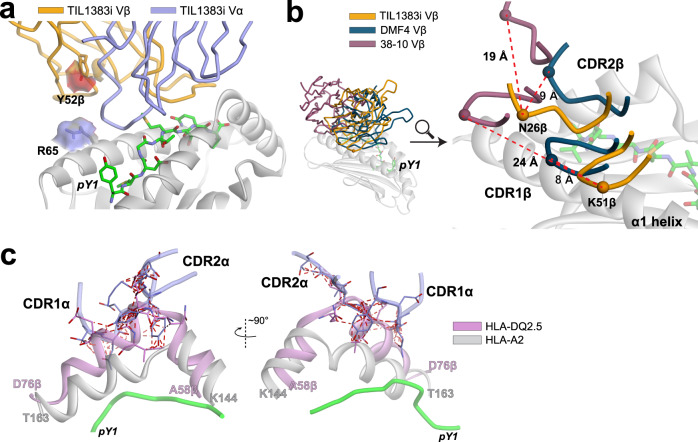
Fig. 4. TIL1383I avoids common or shared TCR-HLA-A2 contacts.
a TIL1383I does not interact with the charge of the Arg65 sidechain on the HLA-A2 α2 helix when bound to Tyr370D/HLA-A2, nor does it use Tyr52 in CDR2β to contact HLA-A2. The solvent-exposed surface areas of the Arg65 and Tyr52β sidechains are shown, emphasizing their lack of participation in the interface. b There are no shared Vβ-HLA-A2 contacts between TIL1383I and two other TCRs that share TRBV10-3 owing to the different placements of the CDR1β and CDR2β loops. c HLA-A2 is not serving as a molecular mimic of a class II protein, as without substantial rearrangements, the binding geometry of TIL1383I would result in significant interatomic steric clashes with the elevated β1 helix of a class II protein, shown by the red lines when the structure of HLA-DQ2.5 is aligned with HLA-A2 in the TIL1383I-Tyr370D/HLA-A2 complex.