Abstract
Cannabis use in pregnancy is associated with adverse perinatal outcomes, which are likely mediated by the placenta. However, the underlying mechanisms and specific vasoactive effects of cannabis on the placenta are unknown. Our objective was to determine the impact of chronic prenatal delta-tetrahydrocannabinol (THC, main psychoactive component of cannabis) exposure on placental function and development in a rhesus macaque model using advanced imaging. Animals were divided into two groups, control (CON, n = 5) and THC-exposed (THC, n = 5). THC-exposed animals received a THC edible daily pre-conception and throughout pregnancy. Animals underwent serial ultrasound and MRI at gestational days 85 (G85), G110, G135 and G155 (full term is ~ G168). Animals underwent cesarean delivery and placental collection at G155 for histologic and RNA-Seq analysis. THC-exposed pregnancies had significantly decreased amniotic fluid volume (p < 0.001), placental perfusion (p < 0.05), and fetal oxygen availability (p < 0.05), all indicators of placental insufficiency. Placental histological analysis demonstrated evidence of ischemic injury with microinfarctions present in THC-exposed animals only. Bulk RNA-seq demonstrated that THC alters the placental transcriptome and pathway analysis suggests dysregulated vasculature development and angiogenesis pathways. The longer-term consequences of these adverse placental findings are unknown, but they suggest that use of THC during pregnancy may deleteriously impact offspring development.
Subject terms: Experimental models of disease, Preclinical research, Translational research
Introduction
Due to recent and widespread legalization at the state level, prenatal cannabis use has more than doubled in the past decade1 and it is now the most common illicit drug used by pregnant individuals2,3. This high prevalence of prenatal cannabis use is in part because approximately half of all pregnancies are unplanned and most women do not recognize they are pregnant until 4 to 6 weeks post conception4. First trimester use of cannabis to treat early pregnancy nausea is common, and coincides with a developmental window (including organogenesis) when the fetus is most vulnerable to adversity. The American College of Obstetricians and Gynecologists5, and the American Academy of Pediatrics6 advise pregnant and lactating patients to abstain from cannabis. Despite these recommendations, many patients continue to use7–9, in part because safety data is lacking and the available literature is insufficient to establish a casual association between cannabis use and negative antenatal outcomes5,10.
The main psychoactive component of cannabis, delta-9-tetrahydrocannabinol (THC), readily crosses the placenta and binds to endocannabinoid receptors in the placenta and key fetal organ systems, leading to concern for detrimental effects on placental and fetal development11–15. Existing studies suggest an increased risk of adverse effects with prenatal cannabis exposure that include preterm birth, stillbirth, small for gestational age infants, and altered offspring neurodevelopment2,13,15–21, but are limited by weak mechanistic understanding and reliance on patient self-reporting. Results are inconsistent among studies, with most studies failing to control for important confounders such as tobacco use. Consequently, the underlying etiology of these adverse outcomes is unclear.
The placenta occupies a critical role in normal maternal–fetal oxygen and nutrient exchange, and altered placental function can result in abnormal fetal development. Rodent placentas exposed to daily intraperitoneal injections of THC exhibited a phenotype characterized by vascular defects including decreased fetal capillary area22. Human data demonstrate that maternal cannabis use is associated with decreased expression of genes involved in placental immune system function, and that several genes organize into co-expression networks that correlate with child anxiety and hyperactivity23.
To address key gaps in the evidence and overcome the limitations of previous human studies, we built upon our novel non-human primate (NHP) model of chronic cannabis exposure via edible THC consumption24,25. Edibles are the second most common mode of cannabis delivery26, especially in non-daily pregnant users27, and are often recommended by dispensaries to pregnant individuals for nausea28. NHPs and humans have similar fetal ontogeny29,30, placental structure29,30, and THC plasma disposition, resulting in observations that are directly translatable to human pregnancies31. A NHP model allows a longitudinal study design, standardization of subject variability, and precise THC dosing to elucidate direct biological consequences of chronic prenatal cannabis exposure while methodically controlling for potential confounders.
In combination, we leveraged our previously developed novel non-invasive MRI methods for the assessment of in utero placental function. We have successfully detected disruption of placental function and development in response to other environmental perturbations, including maternal substance use, in vivo32–36. Placental blood flow is measured using dynamic contrast-enhanced MRI (DCE-MRI)37 and oxygen exchange is quantified through analysis of water T2* values via the blood oxygen level-dependent (BOLD) effect35.
Prenatal cannabis use and potency are increasing, therefore, the need for evidence-driven recommendations on the safety of use during pregnancy is urgent. The primary objective of our study was to evaluate the adverse effects of chronic, prenatal cannabis exposure on placental function and development using a first-in-kind rhesus macaque model of maternal, contemporary THC edible use. Our second objective was to study the effects of prenatal cannabis exposure on placental histology and gene expression to identify mechanisms underlying placental dysfunction.
Materials and methods
Experimental design
All protocols were approved by the Oregon National Primate Research Center (ONPRC) Institutional Animal Care and Use Committee and conformed to all guidelines for humane animal care (IP0001389). Methods are reported in accordance with the ARRIVE guidelines (https://arriveguidelines.org)38. This study used indoor-housed rhesus macaques (n = 10) maintained on a standard chow diet (TestDiet, St. Louis, Missouri). Cookies containing THC (THC edible) were made using research-grade THC obtained directly from the National Institute of Drug Abuse (NIDA)24,25.
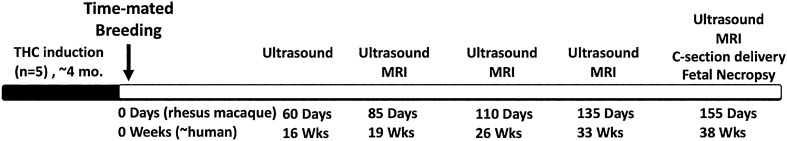
Tap water was available ad libitum. Edibles were administered prior to the animal's daily chow to ensure consumption on an empty stomach and to confirm complete ingestion. Animals were slowly titrated up to 2.5 mg/7 kg/day of THC using published weight-based medical cannabis acclimation recommendations39 approximately 4 months prior to undergoing time-mated breeding as previously published24. Each THC-exposed pregnant animal (n = 5) continued to consume a daily edible of 2.5 mg/7 kg/day throughout pregnancy. All animals (n = 10) underwent Doppler-(D-US) and Contrast-enhanced ultrasound (CE-US) on gestational days 60, 85, 110, 135 and 155 (Fig. 1). Placental MRI consisting of T2* and DCE measurements was performed following ultrasound studies on gestational days 85, 110, 135 and 155. On gestational day 155, after imaging, animals underwent immediate cesarean section delivery with placenta collection and fetal necropsy (Fig. 1). Collected placental tissue was processed in RNAlater for RNA-sequencing, and formalin fixation for histology.
Figure 1.
Study design overview. Timeline of the experimental design indicating that the THC-exposed animals underwent ~ 4 months of THC induction preconception with incremental dosing increase until reaching a dose of 2.5 g/7 kg/day (approximately a heavy medical cannabis dose) that was then maintained throughout pregnancy. The age-matched control group were administered a daily edible without THC in addition to their daily chow. Ultrasound was performed at (G60, term is ~ 168 days), and both US and MRI were performed at G85, G110 and G135, and G155 with immediate cesarean delivery following imaging at G155.
Imaging
Doppler-ultrasound
Ultrasounds were performed by a single sonographer (J.O.L) using image-directed pulsed and color Doppler equipment (GE Voluson 730) with a 5- to 9-MHz sector probe. Animals were sedated by intramuscular administration of 10 mg/kg ketamine (Henry Schein Animal Health®) and maintained on 1.5% isoflurane. Amniotic fluid volume was measured per standard clinical protocol by dividing the uterine cavity into four quadrants, the largest vertical diameter in each quadrant was measured and the sum of the quadrants provided the amniotic fluid index. Doppler waveform measurements for the uterine artery (Uta) and umbilical artery were performed using machine-specific software. The following measurements were obtained: pulsatility index (PI), velocity time integral (VTI), and fetal heart rate (HR) to calculate uterine artery blood flow (cQUta) and placental volume blood flow (cQUV) as previously described33,34,40–43. cQUta was calculated and corrected by maternal weight as: cQUta = VTI × CSA (Uta cross-sectional area) × HR40–43. Placental volume blood flow (cQUV) was calculated as: mean velocity (Vmean) × CSA × 6040–43.
Contrast-enhanced ultrasound
Contrast-enhanced ultrasound (CE-US) was performed using a multiphase amplitude modulation and phase-inversion algorithm on a Sequoia system (Siemens Medical Systems, Mountain View, California) equipped with a 15L8 transducer at a transmit frequency of 7 MHz with a 0.18 mechanical index (MI) and a 55 dB dynamic range with intravenous administration of lipid-shelled octafluoropropane microbubble contrast reagent (Definity; Lantheus Medical Imaging, Billerica, Massachusetts)44. Three replicates of all recordings were obtained and digital imaging data were analyzed as previously described in detail44,45. In brief, regions of interest were drawn over the intervillous space perfused by 1 maternal spiral artery input source. Replenishment kinetic curves were generated in a custom software program, and microvascular flux rate (β) was calculated as the rate of refilling of the vascular space until signal saturation was reached.
Placental MRI
Immediately following ultrasound, MRI was performed on a 3 T Siemens TIM-Trio scanner (Erlangen, Germany) with continuous physiological monitoring as previously published33,34. Following localization of the placenta and acquisition of anatomic, axial 2D multislice multiecho spoiled gradient echo images spanning the entire uterus were acquired at six in-phase echo times for T2* quantification, and was measured with the variable flip angle (VFA) method46. After acquisition of VFA data, 150 3D SPGR volumes were acquired for DCE-MRI with intravenous injection of 0.1 mmol/kg of gadoteridol contrast reagent (Prohance®, Bracco Diagnostics Inc, Princeton, NJ), and with field of view and resolution matched to the VFA images. BOLD and DCE-MRI analyses were performed as previously described32–34,46.
Placental histology
Formalin fixed paraffin-embedded histologic sections were stained with hematoxylin and eosin and reviewed by a single placental pathologist (T.K.M.) blinded to exposure and outcomes. Tissue sections were scored for any signs of infection and classic histologic features of maternal vascular malperfusion, including infarctions and/or accelerated villous maturation47.
Statistical analysis
Mean (± standard deviation) of all fetal biometry and ultrasound measurements were reported at each timepoint and the average changes over time were estimated using linear mixed effects modeling with random intercepts by animal. Treatment group differences in mean maternal age, maternal weight at G60 and G155, fetal birthweight, fetal tissue weight, and total placental weight were assessed using Welch’s t-test. All statistical tests were two-sided and used an alpha of 0.05. Analyses were performed using Stata® version 15.1 (StataCorp, College Station, TX). Linear regression analysis was performed for CE-US data replicates. Differences in DCE-MRI results and BOLD-MRI T2* results were evaluated for a THC effect by repeated measures ANOVA.
Gene expression
RNA isolation and quality assessment
Dissected placental tissue samples (n = 10) in RNAlater (ThermoFisher Scientific) were processed by the OHSU Gene Profiling Shared Resource, where phenol–chloroform extraction was performed followed by RNA isolation using the RNeasy Mini kit (QIAGEN). RNA integrity and size distribution were assessed using a 2100 Bioanalyzer (Agilent Technologies) as previously published34.
RNA sequencing and gene-level differential expression analysis
Isolated placental RNA were sequenced by Novogene. A Poly-A enrichment step was used to select for mRNA molecules followed by fragmentation, reverse transcription, and Illumina-compatible adaptor ligation (PCR and library construction). Libraries were sequenced on a NovaSeq 6000 S4 flow cell (PE150) to generate 20 million paired reads. For bioinformatic analysis, the raw data was filtered and mapped to the rhesus macaque genome. Gene expression levels were quantified using FPKM values to screen for differentially expressed genes (DEGs). ClusterProfiler software48, including Gene Ontology enrichment analysis and KEGG pathway enrichment analysis was performed49–51, on DEGs. Pathway analysis was performed using GO terms and KEGG terms with an adjusted p-value < 0.05.
Ethical approval and consent to participate
The study was approved by the Ethics Committee of the Oregon National Primate Center.
Conference presentation
Presented as oral presentations at the 69th Society of Reproductive Investigation Annual Meeting March 17–19th, 2022 in Denver, Colorado and as poster presentations at the virtual 68th Society of Maternal Fetal Medicine Annual Meeting, February 3–5th, 2022.
Results
THC measures and growth parameters
During the THC induction, average plasma THC concentrations increased by 4.5 ng/mol for each mg/7 kg/day increase in THC (95% CI: 2.6–6.5 ng/mol, p < 0.001). Peak THC levels at the highest THC dosing regimen were within the expected reported contemporary dosing range (e.g., 5–8 ng/mL) in humans 3 h following a similar oral THC dose52,53. Ultrasound measurements of fetal biometry were similar between fetuses exposed to THC and controls across pregnancy (Table 1), and average changes per 25 days of gestation were not significantly different between treatment groups. There were no statistical significance differences in fetal birth weight, maternal weights or fetal gender ratios across treatment groups (Table 2). Fetal tissue weights and tissue:fetal weight ratios were similar between treatment groups, except in THC-exposed fetuses where testicular weight was decreased by approximately half (p < 0.05), and a decreased heart to fetal weight ratio was reported (p < 0.05) (Table 2).
Table 1.
Doppler and contrast-enhanced ultrasound measurements by gestational age timepoint.
| Variable | Group | 60 days | 85 days | 110 days | 135 days | 155 days |
|---|---|---|---|---|---|---|
| Biparietal diameter (cm) | Control | 1.93 ± 0.14 | 2.87 ± 0.22 | 3.86 ± 0.24 | 4.52 ± 0.26 | 4.80 ± 0.07 |
| THC | 1.87 ± 0.13 | 3.03 ± 0.19 | 3.90 ± 0.22 | 4.37 ± 0.30 | 4.72 ± 0.17 | |
| Head circumference (cm) | Control | 7.15 ± 0.27 | 10.67 ± 0.85 | 14.46 ± 0.87 | 16.88 ± 0.76 | 18.00 ± 0.46 |
| THC | 6.81 ± 0.36 | 11.01 ± 0.54 | 14.70 ± 0.64 | 16.69 ± 0.75 | 17.56 ± 0.64 | |
| Abdominal circumference (cm) | Control | 5.61 ± 0.42 | 8.87 ± 0.80 | 11.85 ± 0.39 | 13.54 ± 1.16 | 15.06 ± 0.51 |
| THC | 5.56 ± 0.66 | 8.94 ± 0.51 | 11.65 ± 0.62 | 12.91 ± 0.71 | 14.13 ± 1.04 | |
| Femur length (cm) | Control | 0.80 ± 0.11 | 1.81 ± 0.27 | 2.85 ± 0.26 | 3.44 ± 0.27 | 3.84 ± 0.11 |
| THC | 0.68 ± 0.13 | 1.81 ± 0.25 | 2.72 ± 0.19 | 3.40 ± 0.07 | 3.72 ± 0.29 | |
| Placental volume blood flow (cQUV/kg) | Control | 0.22 ± 0.12 | 0.80 ± 0.35 | 1.47 ± 0.64 | 2.19 ± 0.47 | 2.17 ± 0.70 |
| THC | 0.11 ± 0.04 | 0.79 ± 0.22 | 1.38 ± 0.42 | 1.98 ± 0.42 | 2.14 ± 0.90 | |
| Uterine artery blood flow (cQuta/kg) | Control | 0.14 ± 0.04 | 0.10 ± 0.01 | 0.12 ± 0.04 | 0.12 ± 0.04 | 0.12 ± 0.06 |
| THC | 0.07 ± 0.05 | 0.16 ± 0.12 | 0.18 ± 0.07 | 0.17 ± 0.11 | 0.11 ± 0.10 | |
| Contrast enhanced ultrasound (ms−1) | Control | 0.19 ± 0.04 | 0.22 ± 0.04 | 0.21 ± 0.05 | 0.25 ± 0.11 | 0.22 ± 0.08 |
| THC | 0.23 ± 0.03 | 0.23 ± 0.03 | 0.21 ± 0.02 | 0.19 ± 0.06 | 0.21 ± 0.03 | |
| Umbilical artery pulsatility index (PI) | Control | 2.70 ± 0.46 | 2.09 ± 0.28 | 1.69 ± 0.16 | 1.39 ± 0.31 | 1.09 ± 0.17 |
| THC | 2.41 ± 0.43 | 1.78 ± 0.24 | 1.64 ± 0.41 | 1.32 ± 0.34 | 1.30 ± 0.50 |
CSA (cross section of uterine artery) = π(diameter/2)2.
Vmean (mean velocity) = 0.5 × maximum umbilical vein velocity.
cQuta (uterine artery blood flow) = VTI × CSA × HR adjusted for maternal weight.
cQuv (placental volume blood flow) = Vmean × CSA × 60.
BPD biparietal diameter, AC abdominal circumference, FL femur length, PI pulsatility index, VTI velocity time integral.
Table 2.
Maternal, fetal, and placental weights and demographics.
| Characteristic | Control | THC | p-value |
|---|---|---|---|
| N | 5 | 5 | |
| Maternal age (years) | 10.0 ± 2.3 | 9.9 ± 2.1 | 0.945 |
| Parity | 3.6 ± 0.5 | 2.2 ± 2.2 | 0.226 |
| Maternal weight at G60 days (kg) | 7.53 ± 1.02 | 7.74 ± 0.47 | 0.692 |
| Maternal weight at G155 days (kg) | 8.94 ± 1.20 | 8.95 ± 0.48 | 0.990 |
| Total placental weight (g) | 105.2 ± 17.6 | 105.0 ± 18.7 | 0.984 |
| Fetal measurements | |||
| Fetal sex (male:female) | 2:3 | 3:2 | – |
| Fetal birth weight (kg) | 0.48 ± 0.08 | 0.46 ± 0.07 | 0.676 |
| CRL (cm) | 19.12 ± 1.20 | 19.32 ± 2.08 | 0.859 |
| Right foot (cm) | 7.62 ± 0.66 | 7.3 ± 0.56 | 0.434 |
| AC (cm) | 19.32 ± 2.08 | 11.92 ± 1.57 | 0.930 |
| HC (cm) | 17.18 ± 4.84 | 19.04 ± 0.98 | 0.444 |
| Brain (g) | 53.28 ± 2.71 | 51.92 ± 1.69 | 0.373 |
| Thymus (g) | 1.92 ± 0.59 | 1.45 ± 0.37 | 0.177 |
| Thyroid (g) | 0.35 ± 0.10 | 0.20 ± 0.09 | 0.067 |
| Adrenals (g) | 0.26 ± 0.04 | 0.29 ± 0.13 | 0.598 |
| Pituitary (g) | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.683 |
| Ovaries (g) | 0.09 ± 0.01 (n = 3) | 0.09 ± 0.08 (n = 2) | 0.981 |
| Testes (g)* | 0.13 ± 0.10 (n = 2) | 0.06 ± 0.03 (n = 3) | < 0.05 |
| Kidneys (g) | 2.14 ± 0.31 | 2.18 ± 0.23 | 0.875 |
| Pancreas (g) | 0.35 ± 0.07 | 0.39 ± 0.18 | 0.643 |
| Liver (g) | 12.00 ± 2.23 | 12.32 ± 2.39 | 0.831 |
| Lungs (g) | 9.45 ± 2.76 | 9.07 ± 3.16 | 0.845 |
| Spleen (g) | 0.73 ± 0.17 | 0.57 ± 0.23 | 0.249 |
| Heart (g) | 2.84 ± 0.47 | 2.38 ± 0.62 | 0.222 |
| Body weight (g) | 484.36 ± 76.51 | 463.71 ± 71.21 | 0.670 |
| Heart to weight ratio* | 0.0058 ± 0.0005 | 0.0051 ± 0.0006 | < 0.05 |
CRL crown rump length, AC abdominal circumference, HC head circumference.
Tissue to fetal body weight ratio was not significant for all tissues except heart.
Data are means ± SD. Statistical analysis performed using Welch’s t-test.
*p < 0.05.
Amniotic fluid volume
An expected increase in amniotic fluid volume across pregnancy was observed in control animals (Fig. 2), but a statistically significant decrease in amniotic fluid volume throughout pregnancy was present in THC-exposed animals (p < 0.001).
Figure 2.
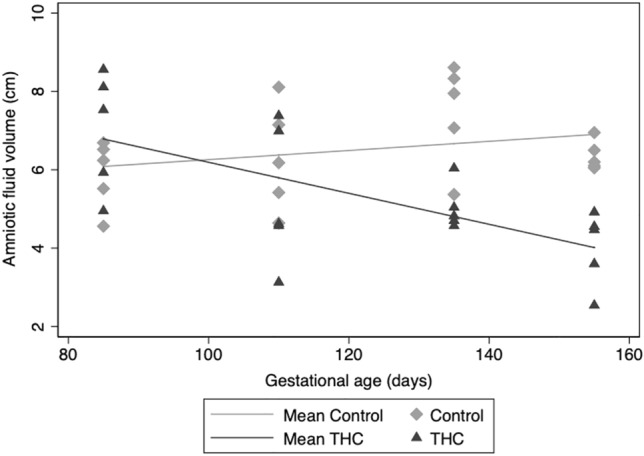
Amniotic fluid volume across gestation. Individual amniotic fluid volume measurements at four gestational time points. With increasing gestation, we observed a significant decrease in average change per 25 days of gestation in amniotic fluid volume in the THC-exposed (n = 5) vs. control (n = 5) pregnancies (p < 0.001).
Placental perfusion and oxygenation
The umbilical artery PI decreased with gestational age as expected and did not deviate from control in any of the THC-exposed animals (Table 1). Quantitative estimation of placental volumetric blood flow in the uterine artery has been previously used by our group and others33,34,40–43,54 to assess maternal-side placental perfusion. cQuta was not significantly different in the THC-exposed group compared to controls across gestation (Table 1). Similarly, the quantitative estimation of blood flow on the fetal side of the placenta33,34,43,54, the cQuv, increased across gestation as expected in both THC-exposed animals and control animals with no significant differences between groups (Table 1). We employed CE-US to visualize and quantify perfusion in the placental intervillous space (IVS). The flux rate constant (β) provides a measure of microvascular resistance and gives an indirect measure of blood flow in the IVS55. In comparing β, there was no difference in vascular impedance between THC-exposed and control animals (Table 1).
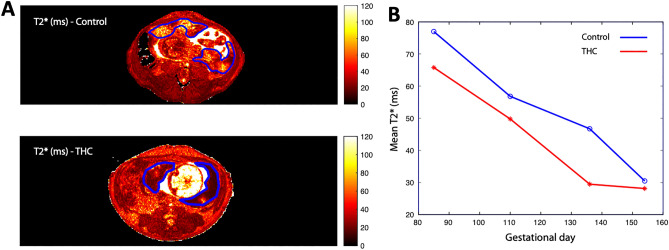
For more comprehensive quantification of blood flow across the entire placenta, DCE-MRI was utilized to assess maternal perfusion of the IVS. Total placental volumetric blood flow was found to be significantly lower (p < 0.05) at G135 and G155 in THC-exposed versus control animals in post hoc comparison (Table 3). Placental oxygen availability was assessed using BOLD-MRI through analysis of quantitative placental T2* values. In control placentas at four timepoints across pregnancy, MR image voxels proximal to spiral artery sources of oxygenated maternal blood are characterized by relatively long T2* (Fig. 3), as previously described35. Concentration of deoxyhemoglobin is higher, reflected by decreased T2*, further from the spiral arteries, secondary to fetal oxygen uptake. There was a statistically significant smaller fraction of large T2* values in THC-exposed animals compared to controls across pregnancy (p = 0.04), demonstrating decreased placental perfusion and fetal oxygen availability in the former (Fig. 3).
Table 3.
Dynamic contrast-enhanced MRI measurements.
| Variable | Group | 60 days | 85 days | 110 days | 135 days | 155 days |
|---|---|---|---|---|---|---|
| Total Placental blood flow (ml/min)a | Control | – | 144.26 ± 51.6 | 169.91 ± 46.17 | 245.27 ± 52.06* | 295.14 ± 36.79* |
| THC | – | 117.166 ± 57.5 | 160.58 ± 76.57 | 169.08 ± 54.35* | 240.27 ± 40.53* | |
| Normalized Placental blood flow (ml blood/ml placenta/min)a | Control | – | 1.20 ± 0.18 | 1.20 ± 0.36 | 1.22 ± 0.03 | 1.26 ± 0.42 |
| THC | – | 1.19 ± 0.35 | 1.11 ± 0.29 | 0.94 ± 0.05 | 1.07 ± 0.25 |
*p < 0.05.
aObtained by DCE-MRI.
Figure 3.
Histogram plot of T2* versus percent of placental voxels displayed for THC-exposed (red) vs. control animals (blue) at G85, G110, G135 and G155. (A) Quantitative T2* maps of placental blood flow of an axial MRI-image at the level of the uterus of a representative control (top) and THC-exposed (bottom) animal with the bi-lobed rhesus macaque placenta outlined in blue. (B) THC-exposed animals had a smaller fraction of large T2* values compared to controls across all time points, demonstrating decreased placental perfusion and fetal oxygen availability in the former. *p = 0.04 for THC effect by repeated measures ANOVA.
Placental histology
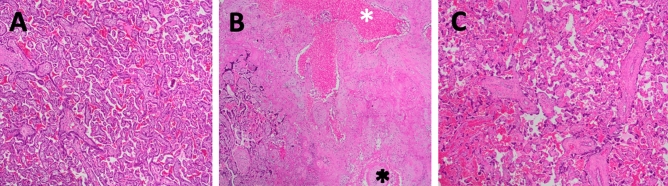
Placental pathology demonstrated increased frequency of microscopic (< 1.0 cm) infarctions and syncytial knots in placentas exposed to THC (4/5, p < 0.05) compared with none in controls (0/5) (Fig. 4). There was no histologic evidence of infection, increase in placental villi maturation or findings of chorangiosis. Placental weights were not different between treatment groups (Table 2).
Figure 4.
Placental microinfarctions associated with prenatal THC exposure. (A) Representative control H&E-stained placental section at gestational day 155 with normal villous and stroma, (B) acute (white asterix) and remote (black asterix) villous infarctions from a THC-exposed placenta, (C) markedly increased syncytial knotting from a THC-exposed placenta. Magnification is ×5.
RNA sequencing
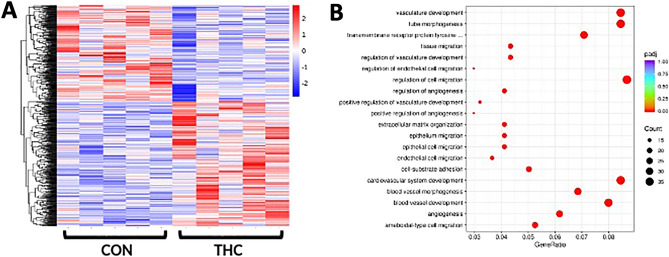
Applying a false discovery rate analysis (p < 0.05), in THC-exposed compared to controls, we identified 426 upregulated genes and 327 downregulated genes (Supplemental Table 1). Hierarchical clustering analysis was performed on differentially expressed genes to identify a distinct gene expression signature in THC-exposed placentas when compared to control placentas (Fig. 5A). In THC-exposed placentas, we further identified 101 genes with p-value < 0.05 and fold-change > 1.5. Comparing THC-exposed placentas to control placentas, pathways with p < 0.001 are shown in Fig. 5B. Gene Ontology (GO) pathway analysis showed significant enrichment in DEGs involved in cytokine binding, regulation of cell migration, cell-substrate adhesion, angiogenesis, and vascular development (Fig. 5B). Similarly, KEGG pathway analysis showed enrichment in DEGs regulating cell migration.
Figure 5.
(A) Bulk placental RNA-sequencing suggests prenatal THC exposure is associated with a clear gene expression signature. RNA-sequencing of rhesus macaque placental tissue at gestational day 155. Heatmap of hierarchical clustering analysis of differentially expressed genes of control (left, n = 5) and THC-exposed (right, n = 5) placental samples. (B) RNA seq pathway analysis. Top significant terms in the Gene Ontology (GO) enrichment analysis of control versus THC-exposed placentas suggest prenatal THC exposure is associated with dysregulated placental vascular development and intracellular signaling, involved in angiogenesis.
Discussion
Chronic prenatal THC exposure significantly diminished amniotic fluid volume, placental perfusion, and fetal oxygen availability throughout pregnancy, associated with altered placental pathology and transcriptome in the NHP. Histologic changes were notable for infarctions and consistent with gene expression enrichment in vascular developmental pathways. These data are all indicative of placental dysfunction resulting from chronic maternal THC use.
Previous animal studies have noted increased22,56,57 or decreased58 placental weight with prenatal cannabis exposure. Interestingly, in our study we did not find any differences in placental weight between treatment groups. This discrepancy may be due to the different route, dosage and timing of cannabis delivery studied. In prior animal studies, THC was often administered by intravenous, intraperitoneal, or oral gavage59,60, whereas our study used THC edibles to recapitulate typical human use28 and achieved plasma THC concentration ranges as previously reported in humans using similar weight-based dosing31.
Prior systematic reviews of human studies18,61 have reported a reduction in birth weight with prenatal cannabis exposure, but did not account for confounding factors such as tobacco and alcohol use. Our study did not find a difference in birth weight between THC exposed and control infants. A systematic review of human studies by Conner et al. adjusted for tobacco use and other confounding factors and also reported no increased risk of low birth weight with cannabis use in pregnancy19.
The clinical implications of our study are that our findings suggest that prenatal cannabis use is associated with placental insufficiency. The combination of significantly decreased placental perfusion and fetal oxygen availability, reduced amniotic fluid volume, and increased placental microinfarctions support a degree of placental dysfunction. The presence of oligohydramnios is a predictor of adverse perinatal outcomes, isolated oligohydramnios in the absence of fetal growth restriction has been associated with increased fetal and neonatal morbidity and mortality62,63. Thus, although fetal weight was maintained, other aspects of fetal development may have been disrupted.
This study was notable for a significant decrease in fetal testicular weight associated with maternal cannabis consumption. Although this finding was in a small sample, it is consistent with our published findings of a significant impact of chronic cannabis use on adult male testicular atrophy25. This is likely in part due to the presence of CB1 and CB2 receptors in both fetal and adult testes, suggesting that cannabis exposure can adversely affect male reproductive health, even in utero. Similar to a rat study of prenatal THC exposure64, we also observed a significant decrease in heart-to-body ratio at birth which may suggest longer term impairments in cardiac function in the offspring.
Our RNA sequencing findings were notable for involvement of angiogenesis and vascular pathways. Taken together with the histologic changes in THC-exposed placentas, these data suggest that prenatal THC exposure not only changes the structure of the placenta but also significantly alters gene expression in pathways regulating cell migration and vascular development. A recently published human cohort of maternal cannabis use reduced expression of placental genes involved in immune system function including type 1 interferon, neutrophil, and cytokine-signaling pathways23. Specifically, this study demonstrated decreased expression of immune genes, including hyperactivity-linked genes IL1B and CXCL8, and S100A823. Similarly, our study was notable for increased expression of IL1RN, an IL1 receptor antagonist, and decreased expression of genes in the S100 protein family.
Our study’s strengths are that it utilizes a translational rhesus macaque model29–31 that imitates typical human cannabis consumption and provides the advantage of precisely measured THC-only exposure, avoiding the toxins of cannabis smoke, while retaining a contemporary, popular prenatal route of THC administration26,28. Other strategies previously used in animal studies59,60 (e.g., oral gavage, intravenous or intraperitoneal injections) may also introduce confounders associated with maternal stress65. The use of a NHP model also minimizes confounding from variables such as gestational age, quantity and timing of THC exposure throughout pregnancy. This model overcomes limitations in human studies including the ability to directly assess the effects of maternal THC consumption, longitudinal in vivo assessment with in utero MRI, and tissue studies. As THC is lipophilic, animals were selected to have a similar pre-pregnancy maternal weight in both treatment groups. All placentas were collected at time of cesarean section delivery prior to onset of spontaneous labor from pregnancies with similar environmental exposures, including diet. This controlled placental collection minimizes potential confounders, such as inflammation, that affect histologic evaluation. The longitudinal in-vivo imaging study design within the same pregnancy permits assessment of changes in placental perfusion and oxygenation across gestation. However, this study was limited by animal cohort size, which did not provide the power to examine fetal sex as a biological variable. With RNA sequencing analysis, our sample size did allow for the detection of DEGs with small effect sizes between treatment groups.
Although we observed placental perturbations from THC exposure, the underlying mechanisms for these observations are not well understood. Maternal environmental exposures ranging from pregnancy nutrition, to opioids and smoking have all been associated with an altered placental transcriptome, including changes linked to neurodevelopmental disorders66–70. THC exerts its effects via receptors of the endocannabinoid system, which plays a critical role in mediating placental and fetal development11–15,71. Endocannabinoid receptors are present in the placenta and major fetal organs, including the brain, starting early in pregnancy72. Although we did not observe a THC effect on fetal brain weights, ongoing work from our group is focused on the impact of prenatal THC on offspring sociobehavior and neurodevelopment. Our RNA sequencing findings reveal mechanistic targets for future investigation to develop interventions or treatments in pregnancies affected by maternal cannabis use. Although contemporary cannabis products used in pregnancy can also contain high doses of cannabidiol (CBD), we chose to study the direct effects of THC only as an initial step given it is the main psychoactive component of cannabis. Future studies will focus on the impact of CBD only and commonly used ratios of THC to CBD.
Summary
The long-term consequences of the adverse placental outcomes we report are unknown, but may impact offspring development. The findings of this study contribute to the limited existing safety data on prenatal THC exposure. At this time, it is important for healthcare providers to counsel pregnant individuals to abstain from THC use until further research is conducted.
Supplementary Information
Acknowledgements
We would like to thank the veterinary and husbandry staff at ONPRC who provided excellent care for the animals used in this study, in particular Dr. Lauren Drew Martin, Dr. Heather Sidener, Travis Hodge, Mike Reusz, and Trent Crowley. Additionally, we would like to thank the Integrated Genomics Laboratory and Gene Profiling Shared Resource, and the Bioanalytical Shared Resource/Pharmacokinetics Core at OHSU. National Institute of Drug Abuse (NIDA) Drug Supply Program.
Author contributions
J.O.L., L.M.B., K.A.G. designed the protocol. J.O.L., M.C.S., T.K.M. conducted investigations. J.J.D.T., R.J.D. and J.A.G. assisted in conduct of work. J.O.L., E.L.S., and V.H.J.R. supervised the work. J.O.L., M.C.S., V.H.J.R., R.J.D., T.K.M. did the data analysis. J.O.L., M.C.S., V.H.J.R., R.J.D. prepared the manuscript. E.R.B. helped with statistical analysis of data. L.M.B., K.A.G., E.L.S. assisted in manuscript preparation. All authors had full access to all the data in the study and read and approved the final version of the manuscript. The corresponding author had final responsibility for the decision to submit for publication.
Funding
This work was funded by NIH/NICHD grant R03 HD097116, NIH/NICHD RSDP K12 HD000849, NIH P51-OD-011092, March of Dimes, Silver Family Innovation Award.
Data availability
The raw and processed RNA-seq datasets used and/or analyzed during the current study are publicly accessible through NCBI Gene Expression Omnibus (GEO) via accession series GSE216112.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Victoria H. J. Roberts and Matthias C. Schabel.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-24401-4.
References
- 1.Brown QL, et al. Trends in marijuana use among pregnant and nonpregnant reproductive-aged women, 2002–2014. JAMA. 2017;317:207–209. doi: 10.1001/jama.2016.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Academies of Sciences, Engineering & Medicine . The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. National Academies of Sciences; 2017. [PubMed] [Google Scholar]
- 3.Martin CE, Longinaker N, Mark K, Chisolm MS, Terplan M. Recent trends in treatment admissions for marijuana use during pregnancy. J. Addict. Med. 2015;9:99–104. doi: 10.1097/adm.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 4.Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N. Engl. J. Med. 2016;374:843–852. doi: 10.1056/NEJMsa1506575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braillon A, Bewley S. Committee opinion no. 722: Marijuana use during pregnancy and lactation. Obstet. Gynecol. 2018;131:164. doi: 10.1097/AOG.0000000000002429. [DOI] [PubMed] [Google Scholar]
- 6.Ryan SA, et al. Marijuana use during pregnancy and breastfeeding: Implications for neonatal and childhood outcomes. Pediatrics. 2018;142:3. doi: 10.1542/peds.2018-1889. [DOI] [PubMed] [Google Scholar]
- 7.Beatty JR, Svikis DS, Ondersma SJ. Prevalence and perceived financial costs of marijuana versus tobacco use among urban low-income pregnant women. J. Addict. Res. Ther. 2012;3:1000135. doi: 10.4172/2155-6105.1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow ND, Compton WM, Wargo EM. The risks of marijuana use during pregnancy. JAMA. 2017;317:129–130. doi: 10.1001/jama.2016.18612. [DOI] [PubMed] [Google Scholar]
- 9.Passey ME, Sanson-Fisher RW, D’Este CA, Stirling JM. Tobacco, alcohol and cannabis use during pregnancy: Clustering of risks. Drug Alcohol Depend. 2014;134:44–50. doi: 10.1016/j.drugalcdep.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Metz TD, Stickrath EH. Marijuana use in pregnancy and lactation: A review of the evidence. Am. J. Obstet. Gynecol. 2015;213:761–778. doi: 10.1016/j.ajog.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Correa F, Wolfson ML, Valchi P, Aisemberg J, Franchi AM. Endocannabinoid system and pregnancy. Reproduction. 2016;152:R191–R200. doi: 10.1530/rep-16-0167. [DOI] [PubMed] [Google Scholar]
- 12.Fride E, et al. The endocannabinoid system during development: Emphasis on perinatal events and delayed effects. Vitam. Horm. 2009;81:139–158. doi: 10.1016/s0083-6729(09)81006-6. [DOI] [PubMed] [Google Scholar]
- 13.Hurd Y, et al. Marijuana impairs growth in mid-gestation fetuses. Neurotoxicol. Teratol. 2005;27:221–229. doi: 10.1016/j.ntt.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Fergusson DM, Horwood LJ, Northstone K, Team AS. Maternal use of cannabis and pregnancy outcome. BJOG Int. J. Obstet. Gynaecol. 2002;109:21–27. doi: 10.1111/j.1471-0528.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- 15.El Marroun H, et al. Intrauterine cannabis exposure affects fetal growth trajectories: The generation R study. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:1173–1181. doi: 10.1097/CHI.0b013e3181bfa8ee. [DOI] [PubMed] [Google Scholar]
- 16.Lo, J. O., Hedges, J. C. & Girardi, G. Impact of cannabinoids on pregnancy, reproductive health and offspring outcomes. Am. J. Obstet. Gynecol. (2022). [DOI] [PMC free article] [PubMed]
- 17.Varner MW, et al. Association between stillbirth and illicit drug use and smoking during pregnancy. Obstet. Gynecol. 2014;123:113. doi: 10.1097/AOG.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunn J, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open. 2016;6:e009986. doi: 10.1136/bmjopen-2015-009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conner SN, et al. Maternal marijuana use and adverse neonatal outcomes. Obstet. Gynecol. 2016;128:713–723. doi: 10.1097/AOG.0000000000001649. [DOI] [PubMed] [Google Scholar]
- 20.Fergusson D, Horwood L, Northstone K. Avon longitudinal study of pregnancy and childhood. Maternal use of cannabis and pregnancy outcome. BJOG. 2002;109:21–27. doi: 10.1111/j.1471-0528.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- 21.Corsi DJ, et al. Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nat. Med. 2020;26:1536–1540. doi: 10.1038/s41591-020-1002-5. [DOI] [PubMed] [Google Scholar]
- 22.Natale BV, et al. Δ9-tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta. Sci. Rep. 2020;10:544. doi: 10.1038/s41598-019-57318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rompala G, Nomura Y, Hurd YL. Maternal cannabis use is associated with suppression of immune gene networks in placenta and increased anxiety phenotypes in offspring. Proc. Natl. Acad. Sci. U S A. 2021 doi: 10.1073/pnas.2106115118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan KS, et al. The effects of delta-9-tetrahydrocannabinol exposure on female menstrual cyclicity and reproductive health in rhesus macaques. FS Sci. 2021;2:287–294. doi: 10.1016/j.xfss.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedges JC, et al. Chronic exposure to delta-9-tetrahydrocannabinol impacts testicular volume and male reproductive health in rhesus macaques. Fertil. Steril. 2022;117:698–707. doi: 10.1016/j.fertnstert.2021.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steigerwald S, et al. Smoking, vaping, and use of edibles and other forms of marijuana among US adults. Ann. Intern. Med. 2018;169:890–892. doi: 10.7326/M18-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young-Wolff KC, et al. Modes of cannabis administration in the year prior to conception among patients in Northern California. Addict. Behav. Rep. 2022;15:100416. doi: 10.1016/j.abrep.2022.100416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickson B, et al. Recommendations from cannabis dispensaries about first-trimester cannabis use. Obstet. Gynecol. 2018;131:1031. doi: 10.1097/AOG.0000000000002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf DP, Thomson JA, Zelinski-Wooten MB, Stouffer RL. In vitro fertilization-embryo transfer in nonhuman primates: The technique and its applications. Mol. Reprod. Dev. 1990;27:261–280. doi: 10.1002/mrd.1080270313. [DOI] [PubMed] [Google Scholar]
- 30.Sibal LR, Samson KJ. Nonhuman primates: A critical role in current disease research. Ilar J. 2001;42:74–84. doi: 10.1093/ilar.42.2.74. [DOI] [PubMed] [Google Scholar]
- 31.Grant KS, Petroff R, Isoherranen N, Stella N, Burbacher TM. Cannabis use during pregnancy: Pharmacokinetics and effects on child development. Pharmacol. Ther. 2018;182:133–151. doi: 10.1016/j.pharmthera.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frias AE, et al. Using dynamic contrast-enhanced MRI to quantitatively characterize maternal vascular organization in the primate placenta. Magn. Reason. Med. 2015;73:1570–1578. doi: 10.1002/mrm.25264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo JO, et al. First trimester alcohol exposure alters placental perfusion and fetal oxygen availability affecting fetal growth and development in a non-human primate model. Am. J. Obstet. Gynecol. 2017;216:302.e301–302.e308. doi: 10.1016/j.ajog.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo JO, et al. Effects of early daily alcohol exposure on placental function and fetal growth in a rhesus macaque model. Am. J. Obstet. Gynecol. 2022;226:130.e131–130.e111. doi: 10.1016/j.ajog.2021.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schabel MC, et al. Functional imaging of the nonhuman primate placenta with endogenous blood oxygen level-dependent contrast. Magn. Reson. Med. 2016;76:1551–1562. doi: 10.1002/mrm.26052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo JO, et al. Novel detection of placental insufficiency by magnetic resonance imaging in the nonhuman primate. Reprod. Sci. 2018;25:64–73. doi: 10.1177/1933719117699704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frias AE, et al. Using dynamic contrast-enhanced MRI to quantitatively characterize maternal vascular organization in the primate placenta. Magn. Reson. Med. 2015;73:1570–1578. doi: 10.1002/mrm.25264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cannabis Clinicians Colorado. Medical Marijuana New Patient Success Guide. http://coscc.org/wp-content/uploads/2016/07/CCC-General-CannabisAsMedicine-2017DRAFT.pdf.
- 40.Konje JC, Kaufmann P, Bell SC, Taylor DJ. A longitudinal study of quantitative uterine blood flow with the use of color power angiography in appropriate for gestational age pregnancies. Am. J. Obstet. Gynecol. 2001;185:608–613. doi: 10.1067/mob.2001.117187. [DOI] [PubMed] [Google Scholar]
- 41.Acharya G, et al. Experimental validation of uterine artery volume blood flow measurement by Doppler ultrasonography in pregnant sheep. Ultrasound Obstet. Gynecol. 2007;29:401–406. doi: 10.1002/uog.3977. [DOI] [PubMed] [Google Scholar]
- 42.Acharya G, Wilsgaard T, Berntsen GK, Maltau JM, Kiserud T. Doppler-derived umbilical artery absolute velocities and their relationship to fetoplacental volume blood flow: A longitudinal study. Ultrasound Obstet. Gynecol. 2005;25:444–453. doi: 10.1002/uog.1880. [DOI] [PubMed] [Google Scholar]
- 43.Frias AE, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152:2456–2464. doi: 10.1210/en.2010-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts VH, et al. Quantitative assessment of placental perfusion by contrast-enhanced ultrasound in macaques and human subjects. Am. J. Obstet. Gynecol. 2016;214(369):e361–368. doi: 10.1016/j.ajog.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts VHJ, et al. Adverse placental perfusion and pregnancy outcomes in a new nonhuman primate model of gestational protein restriction. Reprod. Sci. 2018;25:110–119. doi: 10.1177/1933719117704907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schabel MC, Morrell GR. Uncertainty in T(1) mapping using the variable flip angle method with two flip angles. Phys. Med. Biol. 2009;54:1–8. doi: 10.1088/0031-9155/54/1/n01. [DOI] [PubMed] [Google Scholar]
- 47.Khong TY, et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group consensus statement. Arch. Pathol. Lab Med. 2016;140:698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 48.Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics16, 284–287. 10.1089/omi.2011.0118 (2012). [DOI] [PMC free article] [PubMed]
- 49.Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28:1947–1951. doi: 10.1002/pro.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49:D545–D551. doi: 10.1093/nar/gkaa970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohlsson A, et al. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin. Pharmacol. Ther. 1980;28:409–416. doi: 10.1038/clpt.1980.181. [DOI] [PubMed] [Google Scholar]
- 53.Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin. Chem. 2011;57:66–75. doi: 10.1373/clinchem.2010.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirsch AJ, et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat. Commun. 2018;9:263. doi: 10.1038/s41467-017-02499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts VH, et al. Quantitative assessment of placental perfusion by contrast-enhanced ultrasound in macaques and human subjects. Am. J. Obstet. Gynecol. 2016;214:369.e361–369.e368. doi: 10.1016/j.ajog.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benevenuto SG, et al. Recreational use of marijuana during pregnancy and negative gestational and fetal outcomes: An experimental study in mice. Toxicology. 2017;376:94–101. doi: 10.1016/j.tox.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 57.Carter RC, et al. Alcohol, methamphetamine, and marijuana exposure have distinct effects on the human placenta. Alcohol Clin. Exp. Res. 2016;40:753–764. doi: 10.1111/acer.13022. [DOI] [PubMed] [Google Scholar]
- 58.Chang X, et al. Suppression of STAT3 signaling by Δ9-tetrahydrocannabinol (THC) induces trophoblast dysfunction. Cell Physiol. Biochem. 2017;42:537–550. doi: 10.1159/000477603. [DOI] [PubMed] [Google Scholar]
- 59.Asch R, Smith C. Effects of delta 9-THC, the principal psychoactive component of marijuana, during pregnancy in the rhesus monkey. J. Reprod. Med. 1986;31:1071–1081. [PubMed] [Google Scholar]
- 60.Bailey J, Cunny H, Paule M, Slikker W., Jr Fetal disposition of delta 9-tetrahydrocannabinol (THC) during late pregnancy in the rhesus monkey. Toxicol. Appl. Pharmacol. 1987;90:315–321. doi: 10.1016/0041-008X(87)90338-3. [DOI] [PubMed] [Google Scholar]
- 61.Marchand G, et al. Birth outcomes of neonates exposed to marijuana in utero: A systematic review and meta-analysis. JAMA Netw. Open. 2022;5:2145653–2145653. doi: 10.1001/jamanetworkopen.2021.45653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taneja A, Arora K, Chopra I, Naik SS. Pregnancy outcomes in isolated oligohydramnios during second trimester: A case series. J. Clin. Diagn. Res. 2017;11:Qr01–Qr02. doi: 10.7860/jcdr/2017/27722.10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Figueroa L, et al. Oligohydramnios: A prospective study of fetal, neonatal and maternal outcomes in low-middle income countries. Reprod. Health. 2020;17:19. doi: 10.1186/s12978-020-0854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee K, Laviolette SR, Hardy DB. Exposure to Δ9-tetrahydrocannabinol during rat pregnancy leads to impaired cardiac dysfunction in postnatal life. Pediatr. Res. 2021;90:532–539. doi: 10.1038/s41390-021-01511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hougaard KS, Hansen AM. Enhancement of developmental toxicity effects of chemicals by gestational stress. A review. Neurotoxicol Teratol. 2007;29(4):425–445. doi: 10.1016/j.ntt.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 66.Edlow AG, Guedj F, Sverdlov D, Pennings JLA, Bianchi DW. Significant effects of maternal diet during pregnancy on the murine fetal brain transcriptome and offspring behavior. Front. Neurosci. 2019;13:1335. doi: 10.3389/fnins.2019.01335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shook LL, Kislal S, Edlow AG. Fetal brain and placental programming in maternal obesity: A review of human and animal model studies. Prenat. Diagn. 2020;40:1126–1137. doi: 10.1002/pd.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kawashima A, et al. Effects of maternal smoking on the placental expression of genes related to angiogenesis and apoptosis during the first trimester. PLoS ONE. 2014;9:e106140. doi: 10.1371/journal.pone.0106140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Radhakrishna U, et al. Maternal opioid use disorder: Placental transcriptome analysis for neonatal opioid withdrawal syndrome. Genomics. 2021;113:3610–3617. doi: 10.1016/j.ygeno.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Winterbottom EF, et al. Transcriptome-wide analysis of changes in the fetal placenta associated with prenatal arsenic exposure in the New Hampshire Birth Cohort Study. Environ. Health. 2019;18:100. doi: 10.1186/s12940-019-0535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fuchs Weizman N, et al. Cannabis alters epigenetic integrity and endocannabinoid signalling in the human follicular niche. Human Reprod. 2021;36:1922–1931. doi: 10.1093/humrep/deab104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roncero C, et al. Cannabis use during pregnancy and its relationship with fetal developmental outcomes and psychiatric disorders. A systematic review. Reprod. Health. 2020;17:25. doi: 10.1186/s12978-020-0880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw and processed RNA-seq datasets used and/or analyzed during the current study are publicly accessible through NCBI Gene Expression Omnibus (GEO) via accession series GSE216112.