Abstract
Rationale
The increased mortality and morbidity seen in critically injured patients appears associated with systemic inflammatory response syndrome (SIRS) and immune dysfunction, which ultimately predisposes to infection. Mitochondria released by injury could generate danger molecules, for example, ATP, which in turn would be rapidly scavenged by ectonucleotidases, expressed on regulatory immune cells.
Objective
To determine the association between circulating mitochondria, purinergic signalling and immune dysfunction after trauma.
Methods
We tested the impact of hepatocyte-derived free mitochondria on blood-derived and lung-derived CD8 T cells in vitro and in experimental mouse models in vivo. In parallel, immune phenotypic analyses were conducted on blood-derived CD8 T cells obtained from trauma patients.
Results
Isolated intact mitochondria are functional and generate ATP ex vivo. Extracellular mitochondria perturb CD8+ T cells in co-culture, inducing select features of immune exhaustion in vitro. These effects are modulated by scavenging ATP, modelled by addition of apyrase in vitro. Injection of intact mitochondria into recipient mice markedly upregulates the ectonucleotidase CD39, and other immune checkpoint markers in circulating CD8+ T cells. We note that mice injected with mitochondria, prior to instilling bacteria into the lung, exhibit more severe lung injury, characterised by elevated neutrophil influx and by changes in CD8+ T cell cytotoxic capacity. Importantly, the development of SIRS in injured humans, is likewise associated with disordered purinergic signalling and CD8 T cell dysfunction.
Conclusion
These studies in experimental models and in a cohort of trauma patients reveal important associations between extracellular mitochondria, aberrant purinergic signalling and immune dysfunction. These pathogenic factors with immune exhaustion are linked to SIRS and could be targeted therapeutically.
INTRODUCTION
Trauma is the leading cause of death in individuals under 45 years.1 2 In spite of advances in the acute management of trauma patients, treatment of posttraumatic complications like the systemic inflammatory response syndrome (SIRS) with related sepsis and organ dysfunction, remains the major challenge in critical care medicine.1 2 Severe injury elicits rapid immune responses characterised by aberrant activation of pro-inflammatory pathways in innate immune cells and suppression of adaptive immunity.1–3 Prolonged immune dysfunction is associated with increased susceptibility to secondary infections.2 4 In this regard, previous work has shown that CD8 T cell cytotoxic function is impaired in trauma patients and associated with post-injury multi organ dysfunction syndrome.5 However, the cellular and molecular mechanisms resulting in this immune dysregulation after trauma remain poorly understood.
Mitochondria (MT) are the powerhouse of the cell, generating ATP via oxidative phosphorylation and thus providing energy for cellular metabolism. Previous work has demonstrated that trauma causes a mechanical disruption of cells, resulting in rapid release of MT components like mitochondrial DNA (mtDNA) and formyl peptides, or MT products like ATP, into the circulation. There, these act as danger associated molecular patterns (DAMPs) that can elicit post-injury inflammatory responses.6 7 Such DAMPs as well as pathogen associated molecular patterns derived from infectious agents like bacteria can act together in critically ill patients to impair host defences and to cause SIRS and infection.2 6 The most common pathogens causing nosocomial infection in trauma patients that trigger SIRS include several of the Gram+ bacteria, such as Staphylococcus aureus.8 S. aureus can be frequently found as an innocuous coloniser in healthy human individuals, particularly in nose and skin. However, in immunocompromised patients, these types of bacteria can cause severe infections with high mortality and morbidity.
Extracellular ATP is a well-known danger signal that acts on multiple pathways including inflammation, chemotaxis, inflammasome activation, platelet activation and vascular injury.9 Extracellular purine levels are normally closely regulated by ectonucleotidases that are expressed on various immune cells like T lymphocytes, myeloid and endothelial cells.9 The ectonucleoside triphosphate diphosphohydrolase-1 (ENTPD1), also called CD39, is the ENTPDase family prototype, and scavenges pro-inflammatory ATP and ADP into AMP. AMP is then further degraded into anti-inflammatory adenosine by the ecto-5’-nucleotidase CD73. Ultimately, adenosine is converted into inosine by adenosine deaminase.9
The aim of the present study was to identify potential links between MT release and purinergic signalling that might exist in the setting of experimental trauma and to determine whether these links are also associated with disease outcomes in trauma patients. Importantly, we show that circulating intact whole MT induce dysfunctional CD8 T cell response by increasing CD39, and thereby impact host responses post-injury that in turn predispose to organ damage and bacterial infection.
METHODS
Animals
Male, C57BL/6 wild-type mice (10–12 week-old) were purchased from Taconic and studied in accordance with standard institutional animal welfare guidelines. Procedures were approved by the Institutional Animal Care and Use Committees of Beth Israel Deaconess Medical Center (BIDMC (approved protocol number: 043–2019).
MT isolation and imaging
The liver from one male donor mouse was used to isolate MT using the Mitochondria Isolation Kit (Thermo Fischer Scientific) as previously described.7 10 Liver weights ranged from 1.5 to 1.7 g. Protein concentrations were determined by Bio-Rad DC protein assay reagent (Bio-Rad Laboratories, Hercules, California, USA), using bovine serum albumin as standard.
When performing live-cell imaging, MT were attached to polylysine-coated, glass-bottom chamber slides and stained with mitochondrial membrane potential MitoTracker, reactive oxygen species (ROS)-sensitive dye MitoSOX (both from Thermo Fisher Scientific) and with a cell surface–targeting fluorescent ATP probe (2-2Zn), as previously described.11 Fluorescence imaging was performed with an inverted Leica DMI6000B microscope (Leica Microsystems, Wetzlar, Germany) equipped with a temperature-controlled (37°C) stage incubator (Ibidi, Fitchburg, Wisconsin, USA) and a Leica DFC365 FX camera. Images were captured through ×100 oil objectives (NA 1.4) using tetramethylrhodamine (TRITC) and fluorescein isothiocyanate (FITC) filter sets and LeicaLAS microscope imaging software.
Cell cultures and mitochondrial studies
Murine naive CD8+ T cells were purified from spleen and blood using EasySep Mouse CD8+ T Cell Isolation Kit (StemCell Technologies, Vancouver, British Columbia, Canada) according to the manufacturer’s instructions and were cultured in complete RPMI 1640 medium (Invitrogen, Carlsbad, California, USA), supplemented with 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 1% non-essential amino acids and 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Mononuclear cell viability was assessed by Trypan blue staining and always exceeded 98%. For T cell activation, CD8+ T cells were stimulated with Dynabeads Mouse T-Activator CD3/CD28 (bead/cell ratio 1:1,Thermo Fisher Scientific) and 30 ng/mL interleukin (IL)-2 (R&D Systems, Minneapolis, Minnesota, USA) or were left untreated.
Activated CD8+ T cells were additionally exposed to adenosine (50 μM, Sigma Aldrich, St. Louis, Missouri, USA), ATP (1 mM, Sigma Aldrich) or MT (50 μg/mL) for 24 hours in the absence or presence of apyrase, an ectonucleotidase (10 U/mL, Sigma Aldrich). After 24 hours, flow cytometry was performed, as previously reported.12 Flow cytometry antibodies are listed in the online supplemental material. For intracellular staining of cytokines cells were fixed and permeabilised using the Cytofix/Cytoperm Fixation/Permeabilisation Solution Kit (Thermo Fisher Scientific), according to the manufacturer’s instructions. Cells were acquired on a Cytoflex LX II (Beckman Coulter, Chaska, Minnesota, USA). Data were analysed using Cytexpert analysis software V2.0 (Beckman Coulter). Representative gating strategies are shown in online supplemental figures 1 and 2.
In vivo study
The entire liver from one wild-type mouse (donor) was used to isolate MT and these were injected into five recipient mice. The protein concentration ranged from 1.1 mg/mL to 1.2 mg/mL, given the preparation of 20% of liver, then administered to each mouse. MT pellets were resuspended in Hank’s balanced salt solution (HBSS) with Ca2+ and Mg2+ and injected at 200 μl per mouse injection. The concentration chosen in the current study represents the expected number of MT released after damage of 20% of liver, as predicted during major trauma.
Lung infection studies were conducted as previously described.13 Briefly, S. aureus Rosenbach (ATCC 25923, Manassas, Virginia, USA) was used as the bacterial pathogen. The bacterial culture method resulted in an inoculum of 107–108 colony forming units (CFU), as determined by direct count. To induce bacterial infection in the lung, mice were anaesthetised with ketamine (10 mg/kg) and xylazine (4 mg/kg) by intraperitoneal injection. A 22G needle was carefully placed into the trachea and 80 μl of bacterial suspension was slowly administered.
Mice were sacrificed 24 hours later through the administration of an overdose of anaesthetic (ketamine/xylazine). Blood was collected through terminal cardiac puncture. To collect bronchoalveolar lavage fluid (BALF), lungs were washed with 2.5 mL sterile saline and further processed for determination of CFU counts or for flow cytometry. As previously described,14 the CFU counts were determined by plating 10 μl and 100 μl of BALF on agar plates and quantified the following day. For flow cytometry analysis, alveolar cells were purified after mechanical dissociation and digestion at 37°C for 30 min with digestion buffer containing HBSS with Ca2+ and Mg2+, 0.5 mg/mL Collagenase D (Roche, Milwaukee, Wisconsin’s, USA) and 45 U/mL DNaseI (Millipore Sigma, Burlington, Massachusetts, USA) and further isolated using 40/80% isotonic Percoll gradient centrifugation, as previously described.12 The mononuclear cells obtained from blood and from lung were stained with the antibodies, listed in online supplemental materials.
For histological analysis, lungs were distended by intratracheal administration of optimal cutting temperature media to preserve lung morphology and then snap frozen in liquid nitrogen. The 5 μm sections of the right lung were cut and stained with H&E. Immunohistochemical staining was performed as previously described,15 using the following primary antibodies: rat anti-mouse Ly6G (Cat. No. LS-C36566, LSBio, Seattle, Washington, USA) and rat anti-mouse CD8 (Cat. No. 558733, BD BioSciences, San Jose, California, USA).
Human studies
Peripheral blood samples were obtained prospectively in a randomised manner, as part of the larger study of patients (n=11) entering the trauma centre and control patients undergoing elective surgeries (n=10) at BIDMC, Boston, Massachusetts.
Patients’ and controls’ demographic and detailed clinical information regarding mechanism of injury, hospitalisation stay, intensive care unit (ICU) requirement and need of mechanical ventilation are summarised in online supplemental table 1 for trauma patients and online supplemental table 2 for controls. The clinical data of each patient were collected by the ICU staff and entered prospectively in a REDCap (Research Electronic Data Capture) database. In keeping with current guidelines,16 all trauma patients were further divided into SIRS and non-SIRS subgroups, as based on the vasopressor therapy requirement to maintain a mean arterial pressure (MAP) of 65 mm Hg.
Human peripheral blood mononuclear cells were isolated by density gradient centrifugation and stored at −80°C until further processing, as previously described.17 Flow cytometry was performed using the antibodies listed in online supplemental materials. Comparative analysis was conducted based on the time point of trauma event and presence or absence of SIRS.
Circulating mtDNA was quantified, in blood samples, as previously reported.7 Briefly, real time PCR was carried out using the following primers for human cytochrome B (Forward: 5’-ATGACCCCAATACGCAAAAT-3’, Reverse: 5’-CGAAGTTTCATCATGCGGAG-3’) in order to create standard curves and quantify mtDNA.
Statistics
Statistical analysis was carried out using GraphPad Prism V8.0. Comparisons were made using non-parametric (Mann-Whitney U test) for two-group comparisons. Kruskal-Wallis tests followed by Dunn’s multiple comparison tests were used, when comparing more than two groups. P values <0.05 were considered significant. Figures were created using GraphPad Prism V8.0.
RESULTS
Extracellular MT are functional ex vivo
During cell homeostasis MT serve different functions, including ATP generation via oxidative phosphorylation. However, in a setting of acute trauma, MT components are released extracellularly and can be readily detected in the circulation.7 Also, stimulation of mononuclear cells and other cell types has been noted to cause release of microparticles that can contain MT.18–20 To evaluate whether MT functions are maintained in an ex vivo setting, freshly isolated liver-derived MT were stained with various fluorescent dyes like MitoTracker and MitoSOX as well as with a novel membrane-anchoring ATP probe, 2-2Zn, and then visualised using live-cell imaging.11 These stains indicated that the isolated MT exhibited a stable membrane potential and were functionally active in generating ATP and ROS (figure 1A, online supplemental figure 3A, online supplemental videos 1 and 2). We found that uncoupling of MT by carbonyl cyanide m-chlorophenyl hydrazine resulted in a reduction of MitoTracker fluorescence, indicating loss of MT membrane potential (figure 1A, online supplemental video 1). Furthermore, we observed a decrease in ATP fluorescence after treatment with the ATP removing enzyme apyrase (figure 1A, online supplemental video 2). Notably, MT ROS production, as assessed by MitoSOX fluorescence staining, was increased by the addition of succinate (online supplemental figure 3A, online supplemental video 3), as previously described.21 Meanwhile, ATP release was diminished on addition of succinate (online supplemental figure 3A, online supplemental video 4). These findings suggest that liver-derived MT are stable and functionally active in extracellular microenvironment.
Figure 1.

Extracellular intact whole mitochondria are functional and induce an exhausted immune phenotype in CD8+ T cells in vitro. (A) Liver-derived mitochondria were visualised after isolation using fluorescent MitoTracker and cell-surface targeting ATP indicator 2-2Zn before and after addition of uncoupling agent CCCP (10 μM) and apyrase (10 U/mL) (×100 objective; scale bar, 10 μm). (B–E) CD8+ T cells were left untreated or exposed to CD3/CD28 Dynabeads (control), in the absence or presence of adenosine (ADO) (50 μM), ATP (1 mM) and mitochondria (MT) (50 μg/mL) for 24 hours. Frequency of CD39+ (B) and granzyme B+ cells (C) within the CD8+ cell subset is displayed. Frequency of PD-1+Tim-3+ cells (D) and IL-10+ cells (E) within the CD8+ T cell population is shown. (F–I) CD8+ T cells were cultured in the presence of mitochondria (50 μg/mL) (MT) with or without apyrase (10 U/mL) (MT +APY) in the presence of CD3/CD28 Dynabeads for 24 hours. Frequency of PD-1+ cells (F), PD-1+Tim-3+ cells (G), Tim-3+ cells (H) and IL-10+ cells (I) among CD8+ T cells is shown. Data represent mean±SEM (n=3 per group). P value obtained using Kruskal-Wallis tests followed by Dunn’s multiple comparisons. *p<0.05. CCCP, carbonyl cyanide m-chlorophenyl hydrazine; IL, interleukin; PD-1, programmed cell death 1.
Extracellular MT induce exhausted immune phenotypes in CD8+ T cells in vitro
Adaptive immune responses, particularly involving CD8+ T cells, are dysfunctional in patients with major trauma.4 5 Thus, we continued to study the impact of murine liver MT on murine CD8+ T cells in vitro. We activated CD8+ T cells with CD3/CD28 beads and additionally exposed them to adenosine, ATP or intact MT. As recently described from our group,22 we show that stimulation of CD8+ T cells with anti-CD3/CD28 antibodies induces CD39 expression along with a Tc1 phenotype, as reflected by increased proportion of granzyme B+ cells, compared with untreated cells. Exposure of CD8+ T cells to ATP boosted the frequency granzyme B+ cells. An increase in the frequency of CD39+ cells in response to MT was noted when compared with unstimulated cells (figure 1B,C).
Granzyme B expressing cells increased within the CD39+CD8+ T lymphocyte subset on ATP treatment, while no differences were noted on MT exposure (when compared with untreated cells; online supplemental figure 3B). We further noted decreases in the proportion of interferon (IFN)-γ producing cells within CD8+ T cells in response to adenosine and MT, when compared with anti-CD3/CD28 treatment alone (online supplemental figure 3C).
When stimulated by MT, we noted a significant rise in double positive programmed cell death 1 (PD-1)+Tim-3+ cells within CD8+ lymphocytes (figure 1D), as well as in those cells positive for Tim-3+ only (online supplemental figure 3D), compared with untreated cells. Concomitantly, we observed non-significant changes in the frequency of stimulated CD8+ lymphocytes positive for PD-1, following exposure to ATP or MT, when compared with those CD8+ T cells activated with anti-CD3/CD28 antibodies only (online supplemental figure 3E). Moreover, we noted that the proportion of IL-10+ cells significantly increased within these CD8+ subsets in response to MT (figure 1E).
The effects of the generation of ATP by intact MT ex vivo are abrogated, at least in part, on addition of apyrase. To further confirm that ATP produced by extracellular MT induces the observed phenotype in CD8+ T cells, we noted that the MT-evoked changes in the proportion of PD-1+Tim-3+ cells and single positive Tim-3+ cells within the CD8+ subset were partially abrogated following exposure to apyrase (figure 1G,H). Concomitantly, apyrase supplementation resulted in an increase in PD-1+ cells, compared with CD8+ cells treated with only MT (figure 1F). The increase in the proportion of IL-10+CD8+ cells was partially constrained in the presence of MT and apyrase (figure 1I). Taken together, our data indicate that intact, extracellular ATP-producing MT are able to induce select phenotypic features of exhaustion in CD8+ T cells, as reflected by increased expression of CD39, PD-1 and Tim-3 in vitro.
Circulating CD39+CD8+ T cells exhibit phenotypic features of immune cell exhaustion on administration of liver-derived MT in vivo
Previous work has demonstrated that circulating MT have the ability to activate innate immune cells by binding toll like or formyl peptide receptors, and initiating inflammatory responses.7 10 23 We next sought to determine the effects of circulating intact liver-derived MT on CD8+ T cells obtained from the circulation and lung in a mouse model of MT-induced trauma injury.
We injected wild-type mice with freshly isolated liver-derived MT and analysed peripheral blood and lung-derived lymphomononuclear cell phenotype 24 hours later (figure 2A). Treatment with intact MT resulted in a significant increase in CD8+ cells in the circulation and in a concomitant decrease in these cells in the lung, compared with the control group (figure 2B). The proportion of CD39+CD73− cells was significantly higher among blood-derived CD8+ lymphocytes of MT treated mice, when compared with control mice (figure 2C); whereas the CD39−CD73+ cell fractions did not significantly change (figure 2D). Importantly, the percentage of double positive CD39+CD73+ cells within blood-derived CD8+ T cells significantly increased in mice, exposed to MT (figure 2E). Notably, circulating CD8+ T cells of MT treated mice showed phenotypic features of ‘immune exhaustion’, as reflected by increased frequencies of double positive PD-1+Tim-3+ cells (figure 2F) with trends to higher proportions of lymphocytes positive for cytotoxic T lymphocyte associated protein 4, also referred to as CD152 and a known costimulatory molecule (online supplemental figure 3F).
Figure 2.
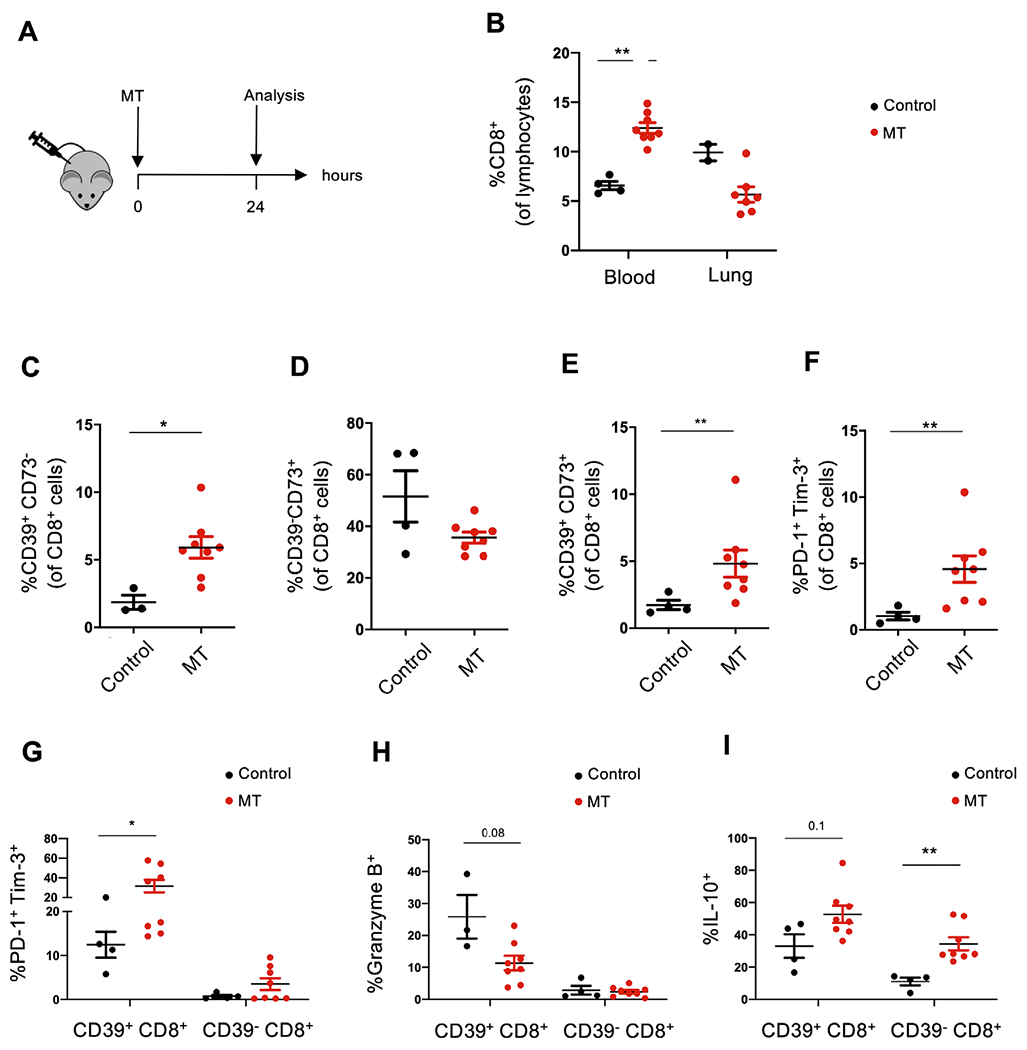
Circulating CD39+CD8+ T cells exhibit features of T cell exhaustion on treatment with liver-derived MT in vivo. (A) Wild-type recipient mice were injected intraperitoneally with liver-derived MT and studied 24 hours later. This MT dose reflects the expected amount of mitochondria released after damage of 20% of liver during major trauma. Blood was collected by cardiac puncture and processed for flow cytometry. (B) Frequencies of CD8+ T cells within peripheral blood-derived and lung-derived lymphocytes in control (untreated) and MT treated mice are shown. Frequencies of CD39+CD73− (C), CD39−CD73+ (D), double positive CD39+CD73+ (E) and PD-1+Tim-3+ cells (F) within blood-derived CD8+ T cells are represented along with the frequency of PD-1+Tim-3+ (G), granzyme B+ (H) and IL-10+ cells (I) among blood-derived CD39+CD8+ and CD39− CD8+ subpopulations. Data represent mean±SEM (n=4 in control group, n=8 in MT treated group). P value obtained using Mann-Whitney U test. *p<0.05; **p<0.01. IL, interleukin; MT, mitochondria; PD-1, programmed cell death 1.
Based on previous work showing that CD39 plays a role in CD8+ T cell immune exhaustion,24 25 we subdivided CD8+ cells into CD39+ and CD39− subpopulations. We noted that blood-derived CD39+CD8+ cells included higher proportions of PD-1+Tim-3+ cells (figure 2G). There were no statistically significant differences between cells obtained from MT treated and untreated mice, although there were trends showing lower frequencies of granzyme-B+ lymphocytes and higher frequencies of IL-10+ cells (figure 2H,I). CD39−CD8+ cells exhibited no significant differences in the percentages of PD-1+Tim-3+ cells and granzyme B+ cells (figure 2G,H). The frequency of IL-10+ cells did increase within the CD39− CD8+ subset (figure 2I).
Collectively, these data indicate that MT injection promotes CD8+ T cell immune responses, and these cells display select features of exhaustion along with altered cytotoxic properties in vivo.
Systemic exposure to intact liver-derived MT results in inflammatory responses and increases the susceptibility to lung infection following instillation of bacteria in vivo
Increased susceptibility to bacterial infection has been observed in patients after major trauma injury.26 To study this, we developed a post-injury pneumonia model and tested how mice are capable of clearing bacterial lung infection after being exposed to intact MT. S. aureus was instilled intra-tracheally 4 hours after MT injection (figure 3A). Histopathological examination revealed that there was more pronounced lung injury on exposure to MT and bacteria (BAC), as indicated by alveolar thickening and recruitment of leucocytes around respiratory alveoli (figure 3B). The lungs obtained from these mice were characterised by a preferential accumulation of Ly6G+ neutrophils, as shown by immunohistochemical and flow cytometry staining (online supplemental figure 4A,B). Analysis of bronchoalveolar lavage fluids showed increased BAC CFU/ml and enhanced recruitment of inflammatory Ly6G+CD11b+ neutrophils into the alveolar space of mice receiving MT and BAC, compared with control mice receiving BAC instillation only (figure 3C,D).
Figure 3.
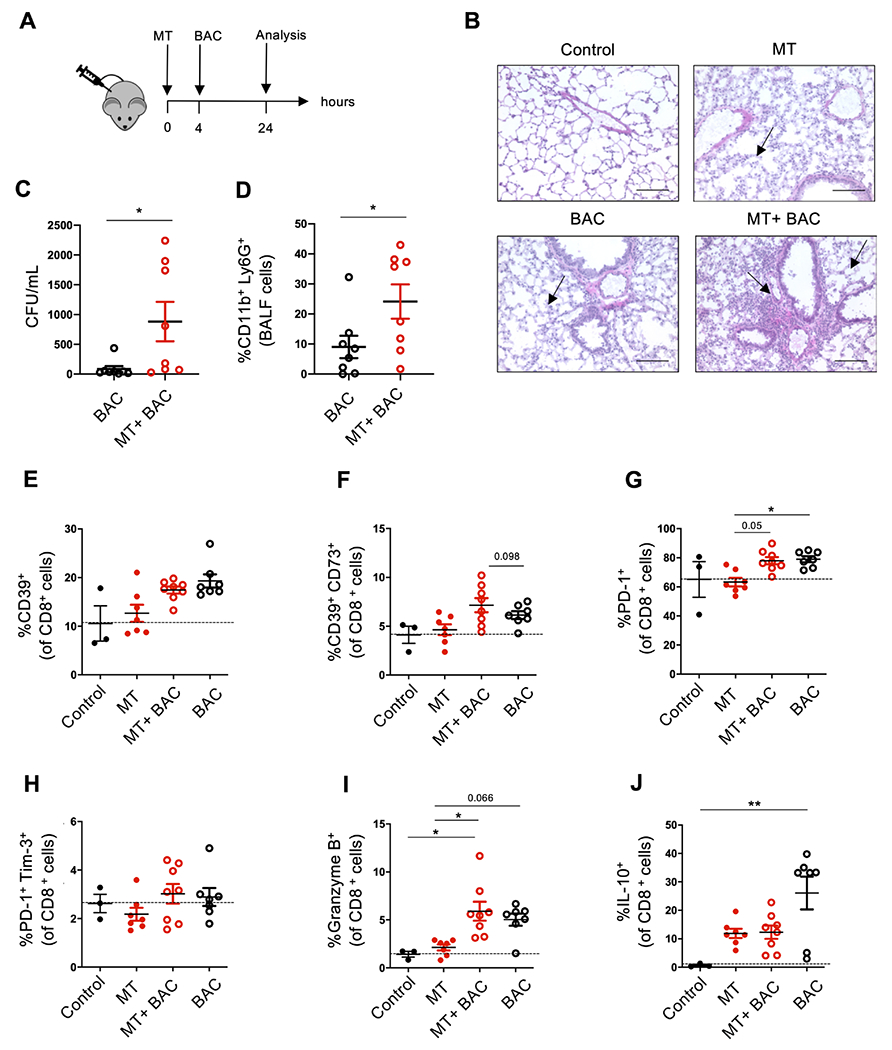
Systemic exposure to intact liver-derived mitochondria results in inflammatory responses and lung infection following instillation of bacteria. (A) Wild-type mice were treated with liver-derived mitochondria (MT) intraperitoneally, followed by intratracheal instillation of Staphylococcus aureus (BAC), after 4 hours. Mice were sacrificed 24 hours later. (B) H&E staining of lung sections in untreated (control), MT, BAC and MT +BAC treated mice. Arrows indicate the alveolar thickening and accumulation of leucocytes in the lung (×20, scale bar: 100 μm). (C–D) Bacterial count in bronchoalveolar lavage (BALF) fluid (C) and frequency of CD11b+Ly6G+ neutrophils within BALF cells (D) in mice treated with BAC only or MT +BAC. (E–J) Flow cytometry analysis of lung-derived mononuclear cells of control (n=4), MT (n=7), BAC (n=8) and MT +BAC (n=8) groups is shown. Frequencies of CD39+ (E), CD39+CD73+ (F), PD-1+ (G), PD-1+Tim-3+ (H), Granzyme B+ (I) and IL-10+ cells (J) within the CD8 subset are shown. Data represent mean±SEM p value obtained using Mann-Whitney U test and Kruskal-Wallis tests followed by Dunn’s multiple comparisons. *p<0.05; **p<0.01. BAC, bacteria; IL, interleukin; PD-1, programmed cell death 1.
Notably, the proportion of CD8+ lymphocytes was reduced in the lung tissue, when MT and BAC were administered in combination, compared with mice receiving MT instillation only (online supplemental figure 4A,C). No differences were observed in the frequency of CD4+ lymphocytes obtained from lung tissues of treated mice (online supplemental figure 4A).
Further characterisation of lung-derived CD8+ T cells revealed that exposure to MT and BAC tended to increase the frequency of the CD39+CD73+ subset (figure 3F), compared with mice treated with BAC only. We also noted that exposure to BAC, alone or in combination with MT, resulted in increased proportions of PD-1+ cells, compared with MT treated mice (figure 3G). Further, MT and BAC administration resulted in an increase in CD8+ T cells with cytotoxic features, as indicated by heightened granzyme B+ cells and non-significant decreases in anti-inflammatory IL-10+ lymphocytes (figure 3I,J), compared with mice administered BAC only. No differences were observed in the frequency of PD-1+Tim-3+ cells and CD39+ cells among lung-derived CD8+ lymphocytes among the four groups (figure 3E,H).
In the subpopulation of CD39+CD8+ T cells, we noted no significant changes in granzyme B+ cells in mice treated with MT +BAC, when compared with BAC only (online supplemental figure 5A). Granzyme B+ cells and IL-10+ cells were increased within CD39−CD8+ T cells in mice administered with BAC alone, when compared with untreated and MT treated mice (online supplemental figure 5B,D). Notably, the frequency of PD-1+Tim-3+ cells within CD39+CD8+ T cells decreased on treatment with MT, MT +BAC or BAC only, when compared with untreated control mice (online supplemental figure 5E). PD-1 Tim-3 expression remained unchanged in CD39−CD8+ T cells (online supplemental figure 5F).
Collectively, these data indicate that administration of MT and BAC instillation boosts CD8+ T cells with cytotoxic features that, despite retaining an immune exhausted phenotype might paradoxically contribute, to the pro-inflammatory microenvironment in the lung.
Human trauma and SIRS are associated with dysfunctional CD8+ T cells
We then assessed the translational relevance of our observed in vitro and in vivo investigations in clinical trauma. The classic paradigm of severe injury describes host responses characterised by early SIRS that is then followed by an immunosuppressive phase.3 To assess the injury-mediated immune response in these two phases, we characterised circulating CD8+ T cells from trauma patients over time (day 0/1 vs day 2/3) and contrasted these with those obtained from control patients about to undergo elective surgery (figure 4A).
Figure 4.

Trauma followed by development of SIRS is associated with circulating dysfunctional CD8+ T cells in human patients. Peripheral blood-derived mononuclear cells were obtained from control patients undergoing elective surgery and from trauma patients on day 0/1 and day 2/3 post injury (n=10 per group). (B) Frequency of CD39+CD73− within blood-derived CD8+ lymphocytes and (C) correlation analysis between mitochondrial DNA and frequency of CD39+CD8+ T cells are shown. (D) Frequency of granzyme B+ cells within CD8+ T cells is displayed. (E) Frequency and representative flow cytometry plots of PD-1+Tim-3+ cells within the CD8+CD39+ subpopulation are also included. (F) Trauma patients were further analysed based on the absence (non-SIRS, n=11) or presence of systemic inflammatory response syndrome (SIRS, n=9). (G–I) Frequencies of CD39+ (G), PD-1+Tim-3+ (H) and granzyme B+ cells (I) within the CD8+ cell population are shown. Data represent mean±SEM p value obtained using Kruskal-Wallis tests followed by Dunn’s multiple comparisons. *p<0.05; **p<0.01; ***p<0.001. mtDNA, mitochondrial DNA; PD-1, programmed cell death 1.
When comparing the controls and trauma patients at day 0/1, there was a trend towards higher frequencies of CD39+CD73− cells (figure 4B). No significant differences were observed in the frequencies of CD73+CD39− and CD39+CD73+ cells among CD8+ T cells between controls and trauma patients, at the different time points (online supplemental figure 6A,B).
In keeping with the findings from Zhang et al,6 we found that trauma patients exhibited higher levels of mitochondrial DAMP (mtDAMPs) in the plasma, when compared with controls (online supplemental figure 6C). Interestingly, correlation analysis revealed associations between circulating mtDNA and percentages of CD39+CD8+ T cells, indicating that circulating mtDAMPs and products might boost CD39 expression on CD8+ T cells (figure 4C).
Importantly, CD8+ T cells obtained from trauma patients at day 2/3 were characterised by decreased frequencies of granzyme B+ cells (figure 4D), consistent with the findings from Hua et al.5 Notably, the CD39+CD8+ cells from trauma patients displayed features of ‘immune exhaustion’, as reflected by significantly higher proportions of double positive PD-1+Tim-3+ cells, particularly at day 2/3 (figure 4E). In addition, the trauma patients exhibited significantly higher levels of PD-1+Tim-3+ cells within the total CD8+ cell subset at day 2/3, when compared with controls (online supplemental figure 6D).
Recent evidence has been provided that pro-inflammatory and immunosuppressive responses can occur simultaneously after trauma.4 27 We therefore analysed the phenotypes of circulating CD8+ T cells derived from trauma patients based on the development of injury-mediated SIRS (figure 4F). We noted trends to increased numbers in the frequency of CD39+CD73− cells among CD8+ lymphocytes obtained from trauma patients with SIRS, when compared with control patients (figure 4G). Notably, these CD8+ T cells obtained from trauma patients with SIRS exhibited increased proportion of PD-1+Tim-3+ cells and trend to decreased frequency of granzyme B+ cells, when compared with controls (figure 4H, I).
Next, we compared the percentage of PD-1+Tim-3+ and granzyme B+ cells in CD39+CD8+ T lymphocytes taken from patients with or without SIRS. We found significant increases in PD-1 and Tim-3 expression in CD39+CD8+ T cell subsets in SIRS patients, but this was not reflected in the CD39−CD8+ T cell subset (online supplemental figure 6F,G). No differences in the frequency of granzyme B+ cells in CD39+ and CD39−CD8+ T cells were noted between the groups (online supplemental figure 6H,I). These findings suggest that dysfunctional CD8+ T cells, putatively characterised by immune exhausted phenotype and reduced cytotoxic capacity, are associated with injury-induced SIRS. This state of immune dysfunction might further increase the susceptibility to infection and thus prolong the critical illness seen in trauma patients (Figure 5).
Figure 5.
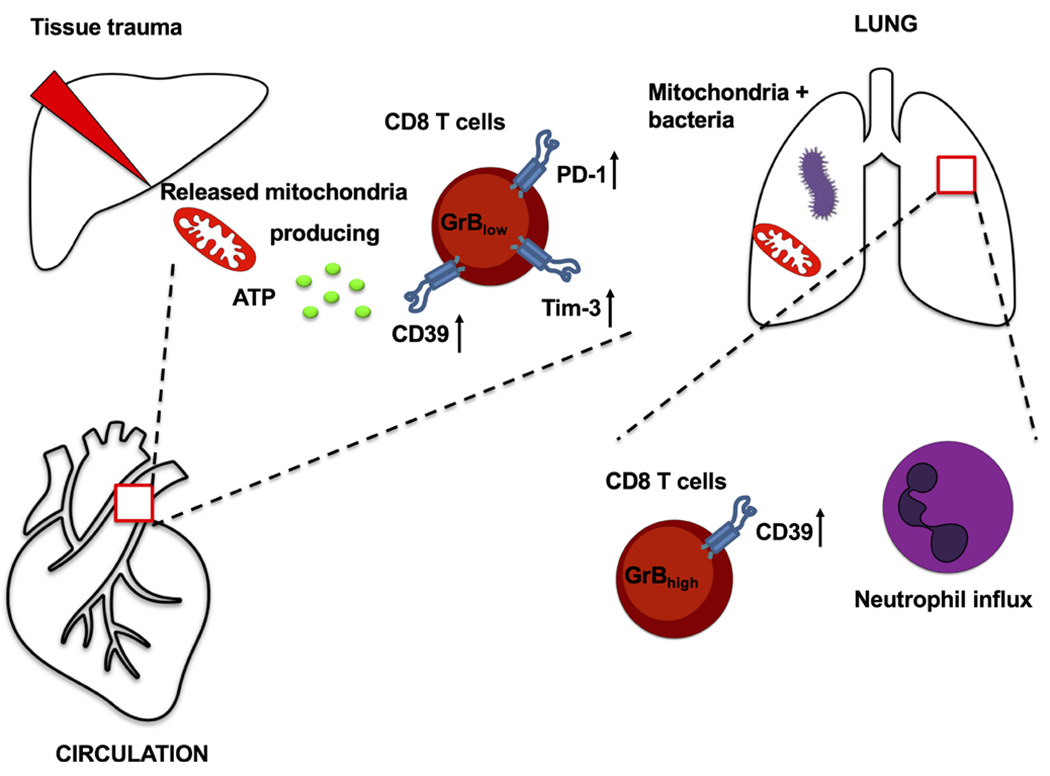
Effects of mitochondria released after tissue trauma on CD8 T cells in vitro and in vivo. GrB, granzyme B; PD-1, programmed cell death 1; Tim-3, T cell immunoglobulin and mucin-domain containing-3.
DISCUSSION
Here we show that intact whole MT are functionally active and can generate ATP in the extracellular milieu. Moreover, these organelles can then induce features of ‘immune exhaustion’ in CD8+ lymphocytes in a paracrine manner in vitro (figure 5). In a MT-induced injury model, circulating intact MT induce CD8+ T cell dysfunction and impair the host immune response following bacterial lung infection (figure 5). The clinical significance of these findings was corroborated in human findings where trauma patients exhibited dysfunctional CD8+ T cell phenotypes, particularly when suffering onset of SIRS, as after traumatic injury.
Using live-cell imaging we have provided evidence that freshly isolated, structurally intact liver-derived MT are capable of producing ROS and ATP in the extracellular environment and that these functions can be modulated by specific agents. Recently, Dache et al described that a certain amount of intact MT circulates in the blood in normal physiological conditions.28 Additional studies reported that whole MT can also be actively excreted from cells in pathophysiological conditions including acute injury, cancer or sepsis.6 29 Recent work from Boudreau et al demonstrated that activated platelets secrete respiratory competent MT, which mediate leucocyte activation via toxic lipid substrates.30 Our previous studies noted that plasma MT DNA levels were about three orders of magnitude higher, immediately after major trauma than in healthy volunteers.7 This raises the possibility that increased free circulating MT might be a significant source of systemic ATP in the blood during major trauma injuries. Interestingly, we observed significant associations between circulating mtDAMPs and increases in CD39+CD8+ T cells, suggesting links between MT and/or derived products to the increases in CD39 expression.
Our in vitro and in vivo work also indicates that the MT-driven immunosuppressive effects on CD8+ T cells might be mediated by ATP-signalling. In this regard, we show that the frequency of CD8+ cells positive for CD39, an ATP hydrolysing ecto-enzyme, was significantly upregulated after mice were exposed to MT. This increase was observed along with higher proportions of CD8+ cells positive for PD-1 and Tim-3, classical T cell exhaustion markers. Several human studies have highlighted that CD39 expression on CD8+ T cells correlates with terminally exhausted T cell function and can be used as an additional marker for immune cell exhaustion in the setting of chronic viral infections and in cancer.24 25 Furthermore, we have previously reported that pathogenic actions of circulating CD39+CD8+ T cells were linked with their ability to produce cytotoxic amounts of IFN-γ.22
However, MT treatment did not significantly change the frequencies of granzyme B+ cells in CD8+ T cells in our in vitro study, suggesting that longer stimulation or additional factors might further modulate the functional properties of CD8+ T cells, resulting in loss and decreases in granzyme B expression. Previous studies have highlighted that granzyme B expression is dependent on T cell clones and might vary between in vitro and in vivo experiments.31 Despite non-significant changes in granzyme B expression in these studies, we observed that intact MT were able to induce features of immune exhaustion, as reflected by high expression of PD-1, Tim-3, CD39 and decreased expression of IFN-γ, in the in vitro study. Future in vivo studies using cell-specific CD39-deficient mice will be essential to further determine the functional and immunomodulatory capacity of MT on CD8 T cells in the setting of trauma.
It has been proposed that continuous production of anti-inflammatory adenosine by CD39 and CD73 contributes to an immunosuppressive micromilieu that can inhibit T cell activation via adenosine-A2A signalling, a mechanism similar to that deployed by T regulatory cells.25 32 We noted, however, that exposure to MT or ATP induced comparable immunosuppressive effects by increasing the proportion of PD-1+Tim-3+ cells among stimulated CD8+ T cells. This effect was abrogated, at least in part, by addition of exogenous apyrase. These data suggest that ATP-producing MT might regulate the expression of these co-inhibitory receptors. Previous studies have indicated that extracellular ATP-driven P2X7 signalling has an impact on T cell differentiation.33 Mouse models using P2×7 deficient mice showed a decreased number of exhausted CXCR5+ expressing CD8+ T cells.34 Further studies are needed to investigate the role of ATP-P2 pathways in regulating the expression of inhibitory receptors on CD8+ T cells.
Although systemic exposure to intact MT resulted in increased frequency of CD39+ and CD39+CD73+ cells both in circulating and lung-derived CD8+ lymphocytes, the functional properties of these two CD8+ subsets were discrete. While lung-derived CD8+ T cells exhibited a Tc-1 like phenotype with increased expression of granzyme B, the CD8+ T cells isolated from the circulation were characterised by a dysfunctional phenotype with impaired cytotoxic features. This functional dichotomy along with the increased pulmonary neutrophil response to BAC seen after exposure to MT DAMPs supports the currently proposed model of injury-mediated immune response. In this model, pro-inflammatory and immunosuppressive responses can occur simultaneously, as also shown in recent studies on circulating leucocyte transcriptome.4 27 Our human data further corroborate the evidence for this, as the development of SIRS was associated with surrogate markers of CD8+ T cell exhaustion in critically ill trauma patients.
In conclusion, we propose that free circulating MT, as released from cells in the setting of trauma, have pathogenetic potential. This is mediated, at least in part, by the generation of extracellular ATP. Consequently, modulation of systemic ATP levels might represent a valuable approach to the treatment of systemic inflammation. This approach might also limit development of immunosuppression, which is seen in patients suffering from major trauma. Both pathways are amenable to targeting using currently available therapeutics.
Supplementary Material
WHAT IS ALREADY KNOWN ON THIS TOPIC
Trauma-induced responses mediated by circulating danger signals, such as those derived from mitochondria, appear to provoke inflammation and predispose to infection. These factors may contribute to the increased mortality seen in critically injured patients. However, the actual mechanisms resulting in immune dysregulation after trauma remain poorly understood and are the focus of this work.
WHAT THIS STUDY ADDS
Extracellular mitochondria are functionally active, generating ATP ex vivo, while exerting immune regulatory effects on CD8 T cells in vitro, and in experimental models in vivo. These effects are linked to alterations of purinergic signalling, as noted with increased expression of CD39 ectonucleotidase on immune cells. Data from clinical studies suggest that complications of trauma are associated with immunosuppressive purinergic responses and elements of CD8 T cell exhaustion, characterised by increases in expression of CD39 and other checkpoint markers.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
To our knowledge, this is the first study that links extracellular mitochondria and ATP generation to aberrant purinergic signalling in the systemic inflammatory response syndrome (SIRS). These events appear to result in CD8 T-cell mediated immune exhaustion, as would be witnessed after trauma, and are associated with SIRS, lung injury and eventually immunosuppression.
Acknowledgements
We would like to thank Ms. Valerie Banner-Goodspeed and Ms. Lauren Kelly of CARE, Department of Anesthesia at Beth Israel Deaconess Medical Center for logistical support.
Funding
The study was supported by the German Research Foundation (DFG TI-988/1-1 to ST-H), National Institutes of Health (HD-098363, GM-116162, and GM-136429 to WJ, R01 DK108894 and R01 DK124408 to MSL and R21 CA164970 to SCR) and the Department of Defense Award W81XWH-16-0464 (to SCR, CJH and LEO).
Footnotes
Competing interests None declared.
Patient consent for publication Consent obtained directly from patient(s).
Ethics approval The study received Institutional Review Board (IRB) approval at Beth Israel Deaconess Medical Center (IRB protocol 2016-P-000144). All patient care and research were conducted in compliance with the Declaration of Helsinki.
All patients provided written informed consent prior to inclusion in the study.
Participants gave informed consent to participate in the study before taking part.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
REFERENCES
- 1.Lord JM, Midwinter MJ, Chen Y-F, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet 2014;384:1455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huber-Lang M, Lambris JD, Ward PA. Innate immune responses to trauma review-article. Nature Immunology 2018;19:327–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med Overseas Ed 2003;348:138–50. [DOI] [PubMed] [Google Scholar]
- 4.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Exp Med 2011;208:2581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hua R, Zhang Y, Chen F, et al. Decreased levels of perforin-positive lymphocytes are associated with posttraumatic complications in patients with major trauma. Injury 2014;45:2089–95. [DOI] [PubMed] [Google Scholar]
- 6.Hauser CJ, Otterbein LE. Danger signals from mitochondrial DAMPs in trauma and post-injury sepsis. Eur J Trauma Emerg Surg 2018;44:317–24. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010;464:104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villavicencio RT, Wall MJ. The pathogenesis of Staphylococcus aureus in the trauma patient and potential future therapies. Am J Surg 1996;172:291–6. [DOI] [PubMed] [Google Scholar]
- 9.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. New England Journal of Medicine 2012;367:2322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itagaki K, Kaczmarek E, Kwon WY, et al. Formyl peptide receptor-1 blockade prevents receptor regulation by mitochondrial danger-associated molecular patterns and preserves neutrophil function after trauma. Crit Care Med 2020;48:e123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledderose C, Liu K, Kondo Y, et al. Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J Clin Invest 2018;128:3583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longhi MS, Vuerich M, Kalbasi A, et al. Bilirubin suppresses Th17 immunity in colitis by upregulating CD39. JCI Insight 2017;2. doi: 10.1172/jci.insight.92791. [Epub ahead of print: 04 May 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itagaki K, Riça I, Zhang J, et al. Intratracheal instillation of neutrophils rescues bacterial overgrowth initiated by trauma damage-associated molecular patterns. J Trauma Acute Care Surg 2017;82:853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GR L D G, de S RWA, et al. Trauma-Induced heme release increases susceptibility to bacterial infection. JCI insight. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowak-Machen M, Schmelzle M, Hanidziar D, et al. Pulmonary natural killer T cells play an essential role in mediating hyperoxic acute lung injury. Am J Respir Cell Mol Biol 2013;48:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer M, Deutschman CS, Seymour CW, et al. The third International consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cahill LA, Guo F, Nguyen J, et al. Circulating factors in trauma plasma activate specific human immune cell subsets. Injury 2020;51:819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konecna B, Park J, Kwon W-Y, et al. Monocyte exocytosis of mitochondrial danger-associated molecular patterns in sepsis suppresses neutrophil chemotaxis. J Trauma Acute Care Surg 2021;90:46–53. [DOI] [PubMed] [Google Scholar]
- 19.Ponomareva AA, Nevzorova TA, Mordakhanova ER, et al. Intracellular origin and ultrastructure of platelet-derived microparticles. J Thromb Haemost 2017;15:1655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tripathi D, Biswas B, Manhas A, et al. Proinflammatory effect of endothelial microparticles is mitochondria mediated and modulated through MAPKAPK2 (MAPK-activated protein kinase 2) leading to attenuation of cardiac hypertrophy. Arterioscler Thromb Vasc Biol 2019;39:1100–12. [DOI] [PubMed] [Google Scholar]
- 21.Chouchani ET, Pell VR, Gaude E, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014;515:431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai A, Moss A, Rothweiler S, et al. NADH oxidase-dependent CD39 expression by CD8(+) T cells modulates interferon gamma responses via generation of adenosine. Nat Commun 2015;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miliotis S, Nicolalde B, Ortega M, et al. Forms of extracellular mitochondria and their impact in health. Mitochondrion 2019;48:16–30. [DOI] [PubMed] [Google Scholar]
- 24.Canale FP, Ramello MC, Núñnez N, et al. CD39 Expression Defines Cell Exhaustion in Tumor-Infiltrating CD8+ T Cells. Cancer Res 2018;78:115–28. [DOI] [PubMed] [Google Scholar]
- 25.Gupta PK, Godec J, Wolski D, et al. Cd39 expression identifies terminally exhausted CD8+ T cells. PLoS Pathog 2015;11:e1005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pories SE, Gamelli RL, Mead PB, et al. The epidemiologic features of nosocomial infections in patients with trauma. Arch Surg 1991;126:97–9. [DOI] [PubMed] [Google Scholar]
- 27.Chen T, Delano MJ, Chen K. A roadmap from single-cell transcriptome to patient classification for the immune response to trauma. JCI Insight. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Amir Dache Z, Otandault A, Tanos R, et al. Blood contains circulating cell-free respiratory competent mitochondria. Faseb J 2020;34:3616–30. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez A-M, Nakhle J, Griessinger E, et al. Intercellular mitochondria trafficking highlighting the dual role of mesenchymal stem cells as both sensors and rescuers of tissue injury. Cell Cycle 2018;17:712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boudreau LH, Duchez A-C, Cloutier N, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 2014;124:2173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelso A, Costelloe EO, Johnson BJ, et al. The genes for perforin, granzymes A-C and IFN-gamma are differentially expressed in single CD8(+) T cells during primaiy activation. Int Immunol 2002;14:605–13. [DOI] [PubMed] [Google Scholar]
- 32.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007;204:1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanhainen KM, Jameson SC, da Silva HB, da SHB. Self-Regulation of memory CD8 T cell metabolism through extracellular ATP signaling. Immunometabolism 2019;1. doi: 10.20900/immunometab20190009. [Epub ahead of print: 23 07 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borges da Silva H, Beura LK, Wang H, et al. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8+ T cells. Nature 2018;559:264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.


