Abstract
Background
Current guidelines for the therapeutic monitoring of vancomycin recommend dosing based on the area under the concentration-time curve (AUC) to achieve clinical efficacy while reducing nephrotoxicity. Although a wide range of nephrotoxicity thresholds have been reported, few studies have documented clinical outcomes based on AUC-guided vancomycin dosing in Korea.
Objective
The aim of the study was to evaluate whether a relationship exists between AUC and treatment outcomes in vancomycin treated patients in methicillin-resistant Staphylococcus aureus bacteremia. Furthermore, this study tries to estimate AUC threshold for treatment failure and nephrotoxicity.
Methods
The records of adult patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin for ≥72 hours without dialysis between April 2013 and April 2021, were reviewed retrospectively. Treatment success was defined as defervescence and blood culture sterilization by day 7. Nephrotoxicity was defined as an increase in serum creatinine levels ≥0.3 mg/dL or a 50% increase from baseline on 2 consecutive days. Bayesian estimation was used to predict individual vancomycin AUC. Both classification and regression tree and receiver operating characteristic curve analyses were performed to estimate the optimal AUC thresholds for vancomycin efficacy and nephrotoxicity.
Results
Of 118 patients, 61 (51.7%) experienced treatment failure and 42 (35.6%) developed acute kidney injury. The vancomycin AUC threshold for predicting acute kidney injury was 615.0 mg· hr/L. In the multivariate analysis, AUC ≥615.0 mg· hr/L was a significant risk factor for nephrotoxicity (adjusted odds ratio [aOR] = 5.24; 95% CI, 1.8–14.65). The lower threshold for treatment failure was not defined because it was not statistically significant. Risk factors for treatment failure included low body mass index (aOR = 0.82; 95% CI, 0.70–0.96), severity of acute illness represented by complicated infection (aOR = 77.56; 95% CI, 16.7–359.4) and comorbidities, such as solid organ tumors (aOR = 6.61; 95% CI, 1.19–36.81) and cerebrovascular disease (aOR = 6.05; 95% CI, 1.17–31.23).
Conclusions
Although AUC-guided vancomycin dosing was associated with a reduced risk of acute kidney injury, its ability to predict clinical outcomes was modest. Further studies are needed to define the AUC therapeutic range to maximize efficacy and minimize nephrotoxicity. (Curr Ther Res Clin Exp. 2023; 83:XXX–XXX)
Key words: area under the concentration-time curve, bacteraemia, methicillin-resistant Staphylococcus aureus, nephrotoxicity, vancomycin
Introduction
Vancomycin is among the most widely prescribed antibiotics for the treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections. However, the optimal dosing strategy for vancomycin remains undefined, and its narrow therapeutic window necessitates therapeutic drug monitoring (TDM) to ensure clinical efficacy and avoid nephrotoxicity.1,2 The first consensus review of TDM for vancomycin was published in 2009, and that recommended maintaining serum trough concentrations between 15 and 20 mg/L as a surrogate marker to achieve an area under the curve (AUC) to MIC ratio ≥400, assuming an MIC ≤1 mg/L for serious MRSA infections.2,3
However, recent data by Neely et al4 suggested that nearly 50% to 60% of patients achieve an AUC ≥400 mg· hr/L at trough concentrations <15 mg/L, suggesting that trough concentration is a poor surrogate for AUC. Chavada et al5 also showed that 26.7% of patient have an AUC ≥400 mg· hr/L while demonstrating trough levels below 15 mg/L. Furthermore, whether an increase in trough concentration is associated with improvements in clinical outcomes remains controversial.6,7 Thus, the newly released 2020 Infectious Disease Society of America guidelines recommend monitoring AUC/MIC for optimal and accurate vancomycin dosing, utilizing Bayesian software programs to estimate the AUC.8 A few studies have adopted this method to analyze vancomycin AUC and clinical outcomes, but their conclusions have been inconsistent.6,7,9, 10, 11, 12, 13 Jung et al11 demonstrated that a low AUC/MIC (<430 by Etest; <398.5 by broth microdilution [BMD]) was a significant risk factor for treatment failure in MRSA bacteremia, and Lodise et al12 also described that achievement of AUC/MIC target was associated with 2-fold decrease in treatment failure. A meta-analysis by Tsutsuura et al7 used an AUC/MIC cutoff of 400 (400 ±15%) to show that high AUC/MIC values had lower treatment failure rates (odds ratio [OR] = 0.28; 95% CI, 0.18–0.45). In contrast, a recent prospective observational study by Lodise et al10 and meta-analysis by Tongsai et al6 found no clear relationship between vancomycin AUC and treatment efficacy. Regarding nephrotoxicity, a number of published reports have agreed that the risk of acute kidney injury (AKI) increases as vancomycin AUC increases, especially when the daily AUC exceeds 700 mg· hr/L.4,14,15 A different AUC threshold above 650 mg· hr/L was provided from a meta-analysis by Aljefri et al.16 Although consensus guidelines suggest that vancomycin AUCs should be between 400 and 600 to ensure clinical efficacy and safety, the AUC threshold associated with treatment success and nephrotoxicity has yet to be clearly defined.5,11,13,15 Therefore, the aim of this study was to evaluate the relationship between AUC and treatment outcomes and to further estimate AUC threshold for treatment failure and nephrotoxicity in patients with MRSA bacteremia receiving vancomycin treatment. Additionally, we analyzed potential risk factors affecting clinical outcomes and nephrotoxicity.
Participants and Methods
Study design and setting
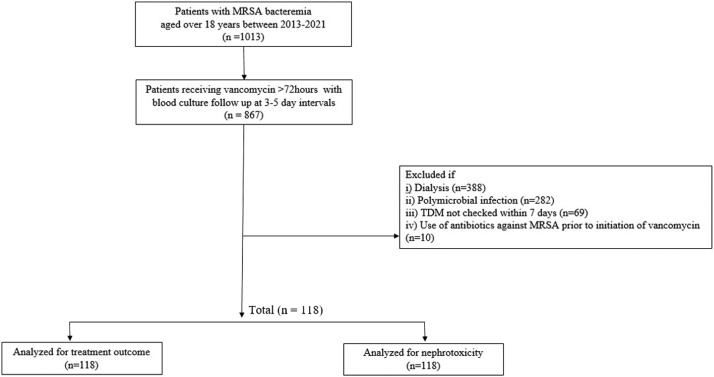
This was a retrospective single-center cohort study performed at Ajou University Hospital, a 1187-bed tertiary hospital in Kyunggi province. Patients older than age 18 years admitted between April 1, 2013, and April 1, 2021, presenting with their first episode of MRSA bacteremia were screened. The inclusion criteria for this study were as follows: patients who received intravenous vancomycin for at least 3 days and patients who underwent follow-up blood cultures at 3- to 5-day intervals. The exclusion criteria were as follows: patients on dialysis, those who had not been checked for TDM within 7 days of starting vancomycin, patients with polymicrobial infection, and patients treated with antibiotics against MRSA before initiation of vancomycin (Figure 1). This study was conducted in accordance with the Declaration of Helsinki, and was approved by the Institutional Review Board of Ajou University School of Medicine (No. AJOUIRB-DB-2022-367).
Figure 1.
Flow chart outline of the study design. MRSA = methicillin-resistant Staphylococcus aureus; TDM = therapeutic drug monitoring.
Data collection and definitions
Demographic characteristics, comorbidities, and primary infection sites were reviewed from the electronic medical records. Vital signs and laboratory findings were assessed to quantify the severity of illness using the Pitts bacteremia score, which was calculated using the worst parameters within 48 hours before or on the day of the first positive blood culture. Charlson's weighted index of comorbidities (WIC) was determined after inspecting the patients’ medical records. The use of potential nephrotoxic drugs, including contrast dye, piperacillin/tazobactam, aminoglycosides, amphotericin B, colistin, furosemide, thiazides, vasopressors, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, nonsteroidal anti-inflammatory drugs, tacrolimus, cyclosporine, and cisplatin, was identified from medication administration records.
Treatment success was defined as resolution of bacteremia and fever within 7 days of vancomycin therapy. The treatment failure group was composed of patients who did not meet these criteria, died within 30 days, or had a positive follow-up culture more than 14 days after a negative follow-up culture. All-cause mortality was defined as death that occurred during hospitalization.
Vancomycin-induced nephrotoxicity was defined as an increase in serum creatinine levels ≥0.3 mg/dL or a 50% increase from baseline on 2 consecutive days without an alternative explanation, according to the Acute Kidney Injury Network and the Kidney Disease: Improving Global Outcomes criteria.17 The duration of bacteremia was determined as the time period from the day of the first positive blood culture to the last positive follow-up blood culture. Patients with metastatic infection, implanted prostheses, and endocarditis were classified as having complicated bacteremia.
Estimation of AUC/MIC
Steady-state vancomycin trough concentrations were used to estimate the AUC/MIC ratio. According to our institutional dosing guidelines, we monitor the vancomycin concentration before the fourth or fifth dose in all patients, assuming this point as a steady-state condition. Using a priori pharmacokinetic parameters of previous population pharmacokinetic model, posteriori Bayesian estimation was used to predict steady-state vancomycin AUC, utilizing dose individualization software (Capcil software, Simkin Inc, Gainesville, Florida). Based on a large surveillance program that identified 95% of MRSA isolates as having MICs ≤1 mg/dL,18 only the AUC was used.
Statistical methods
Statistical comparisons were made using the χ2 test, Fisher exact test, or Student t test, as appropriate. Classification and regression tree (CART) and receiver operating characteristic (ROC) curve analyses were performed to estimate the AUC thresholds for vancomycin efficacy and nephrotoxicity. Logistic regression analysis was used to predict the risk factors for treatment failure and AKI. All statistical analyses were performed using IBM SPSS statistics version 25 (IBM-SPSS Inc, Armonk, New York) and MedCalc Statistical Software version 19.2.6 (MedCalc Software Ltd, Ostend, Belgium). Statistical significance was set at P < 0.05.
Results
Demographic and clinical characteristics
A total of 118 patients who met the inclusion criteria were included in the study. The baseline demographic and clinical characteristics of the cohort are shown in Tables 1 and 2 according to clinical outcome and vancomycin-induced nephrotoxicity, respectively. Of the 118 patients, 61 (51.7%) experienced treatment failure, and 42 (35.6%) developed nephrotoxicity. The number of male patients was 73 (61.9%), and the mean (SD) age was 63.50 (16.29) years. More than half of the patients (51.7%) were older than age 65 years. Diabetes was the most common comorbidity (34.7%), followed by cardiovascular disease (26.3%), cerebrovascular disease (21.2%), and solid organ tumors (20.3%).
Table 1.
Clinical characteristics of patients with methicillin-resistant Staphylococcus aureus bacteremia according to vancomycin treatment outcomes.
| Characteristic | Total patients (N = 118) | Vancomycin treatment outcome |
P value | |
|---|---|---|---|---|
| Success (n = 57) | Failure (n = 61) | |||
| Age ≥65 y* | 61 (51.7) | 26 (45.6) | 35 (57.4) | 0.201 |
| Male sex* | 73 (61.9) | 35 (60.3) | 38 (63.3) | 0.921 |
| Body mass index† | 23.66 (4.09) | 24.20 (4.29) | 23.16 (3.8) | 0.167 |
| Charlson's weighted index of comorbidity† | 2.61 (2.32) | 2.04 (1.71) | 3.15 (2.68) | 0.008 |
| Underlying conditions* | ||||
| Diabetes | 41 (34.7) | 21 (36.8) | 20 (32.8) | 0.644 |
| Cardiovascular disease | 31 (26.3) | 12 (21.1) | 19 (31.1) | 0.213 |
| Chronic liver disease | 12 (10.2) | 6 (10.5) | 6 (9.8) | 0.901 |
| Chronic lung disease | 11 (9.3) | 4 (7.0) | 7 (11.5) | 0.405 |
| Cerebrovascular disease | 25 (21.2) | 9 (15.8) | 16 (26.2) | 0.165 |
| Chronic kidney disease stage 3 to 5 | 7 (5.2) | 3 (5.3) | 4 (6.6) | 1.000 |
| Rheumatic disease | 10 (8.5) | 4 (7.0) | 6 (9.8) | 0.744 |
| Solid organ tumor | 24 (20.3) | 6 (10.5) | 18 (29.5) | 0.010 |
| Hematologic malignancy | 6 (5.1) | 4 (7.0) | 2 (3.3) | 0.428 |
| Immunosuppressive agents | 14 (11.9) | 5 (8.8) | 9 (14.8) | 0.315 |
| Source of infection* | 0.486 | |||
| Catheter-related bloodstream infection | 33 (28.0) | 21 (36.8) | 12 (19.7) | 0.038 |
| Bone and joint infection | 28 (23.7) | 12 (21.1) | 16 (26.2) | 0.509 |
| Skin and soft tissue infection | 21 (17.2) | 10 (17.5) | 11 (18.0) | 0.945 |
| Infective endocarditis | 5 (4.2) | 1 (1.8) | 4 (6.6) | 0.366 |
| Central nervous system infection | 1 (0.8) | 0 (0.0) | 1 (1.6) | 1.000 |
| Pneumonia | 10 (8.5) | 5 (8.8) | 5 (8.2) | 1.000 |
| Primary sepsis | 10 (8.5) | 4 (7.0) | 6 (9.8) | 0.744 |
| Other | 10 (8.5) | 4 (7.0) | 6 (9.8) | 0.744 |
| Septic shock* | 63 (53.4) | 25 (43.9) | 38 (62.3) | 0.045 |
| Pitt bacteremia score† | 2.31 (2.26) | 1.72 (1.81) | 2.87 (2.51) | 0.005 |
| Complicated infection* | 64 (54.2) | 11 (19.3) | 53 (86.9) | < 0.001 |
| Area under the concentration-time curve,† mg· hr/L | 581.50 (220.78) | 557.57 (188.50) | 603.86 (246.65) | 0.253 |
| Trough concentration,† mg/L | 15.49 (7.51) | 15.20 (6.52) | 15.76 (8.37) | 0.682 |
| Area under the concentration-time curve cut-off <373.5 mg· hr/L* | 15 (12.7) | 5 (8.8) | 10(16.4) | 0.273 |
| Bacteremia duration,† d | 5.61 (6.14) | 1.84 (2.04) | 9.13 (6.60) | < 0.001 |
| Mean time of blood culture follow-up,† d | 3.21 (1.99) | 3.02 (1.25) | 3.41 (1.13) | 0.076 |
| Development of acute kidney injury* | 42 (35.6) | 15 (26.3) | 27 (44.3) | 0.042 |
Values are presented as n (%).
Values are presented as mean (SD).
Table 2.
Clinical characteristics of patients with methicillin-resistant Staphylococcus aureus bacteremia according to vancomycin-induced nephrotoxicity.
| Characteristic | Total patients (N = 118) | >Nephrotoxicity |
P value | |
|---|---|---|---|---|
| No (n = 76) | Yes (n = 42) | |||
| Age ≥65 y* | 61 (51.7) | 34 (44.7) | 27 (64.3) | 0.042 |
| Male sex* | 73 (61.9) | 47 (61.8) | 26 (61.9) | 0.995 |
| Body mass index† | 23.66 (4.09) | 23.83 (4.09) | 23.37 (4.13) | 0.559 |
| Charlson's weighted index of comorbidity† | 2.61 (2.32) | 2.26 (2.05) | 3.24 (2.66) | 0.028 |
| Underlying conditions* | ||||
| Diabetes | 41 (34.7) | 25 (32.9) | 16 (38.1) | 0.570 |
| Cardiovascular disease | 31 (26.3) | 16 (21.1) | 15 (35.7) | 0.083 |
| Chronic liver disease | 12 (10.2) | 5 (6.6) | 7 (16.7) | 0.080 |
| Chronic lung disease | 11 (9.3) | 8 (10.5) | 3 (7.1) | 0.744 |
| Cerebrovascular disease | 25 (21.2) | 14 (18.4) | 11 (26.2) | 0.323 |
| Chronic kidney disease 3 to 5 | 7 (5.2) | 1 (1.3) | 6 (14.3) | 0.008 |
| Rheumatic disease | 10 (8.5) | 6 (7.9) | 4 (9.5) | 0.742 |
| Solid organ tumor | 24 (20.3) | 12 (15.8) | 12 (28.6) | 0.099 |
| Hematologic malignancy | 6 (5.1) | 5 (6.6) | 1 (2.4) | 0.420 |
| Any use of immunosuppressive agents | 14 (11.9) | 8 (10.5) | 6 (14.3) | 0.563 |
| Source of infection* | ||||
| Catheter-related bloodstream infection | 33 (28.0) | 24 (31.6) | 9 (21.4) | 0.240 |
| Bone and joint infection | 28 (23.7) | 25 (32.9) | 3 (7.1) | 0.002 |
| Skin and soft tissue infection | 21 (17.2) | 12 (15.8) | 9 (21.4) | 0.443 |
| Infective endocarditis | 5 (4.2) | 0 (0.0) | 5 (11.9) | 0.005 |
| Central nervous system infection | 1 (0.8) | 0 (0.0) | 1 (2.4) | 0.356 |
| Pneumonia | 10 (8.5) | 5 (6.6) | 5 (11.9) | 0.325 |
| Primary sepsis | 10 (8.5) | 5 (6.6) | 5 (11.9) | 0.325 |
| Other focus | 10 (8.5) | 5 (6.6) | 5 (11.9) | 0.325 |
| Septic shock* | 63 (53.4) | 37 (48.7) | 26 (61.9) | 0.168 |
| Pitt bacteremia score† | 2.31 (2.26) | 1.91 (1.77) | 3.05 (2.84) | 0.021 |
| Complicated infection* | 64 (54.2) | 35(46.1) | 29 (69.0) | 0.016 |
| Area under the concentration-time curve,† mg· hr/L | 581.50 (220.78) | 561.82 (160.85) | 698.52 (264.56) | < 0.001 |
| Trough concentration,† mg/L | 15.49 (7.51) | 13.56 (5.75) | 18.99 (8.99) | 0.001 |
| Bacteremia duration,† d | 5.61 (6.14) | 4.97 (6.01) | 6.76 (6.29) | 0.137 |
| Mean time of blood culture follow-up,† d | 3.21 (1.99) | 3.13 (1.19) | 3.38 (1.21) | 0.281 |
| Concomitant use of nephrotoxic agent* | 98 (83.1) | 57 (75.0) | 41 (97.6) | 0.002 |
Values are presented as n (%).
Values are presented as mean (SD).
Catheter-related infection was the most common source of MRSA bacteremia (28.0%), and 5 patients (4.2%) were diagnosed with infective endocarditis. Other sources of infection included deep neck infection (2 cases), prostatitis with urinary tract infection (5 cases), and intra-abdominal infection (3 cases).
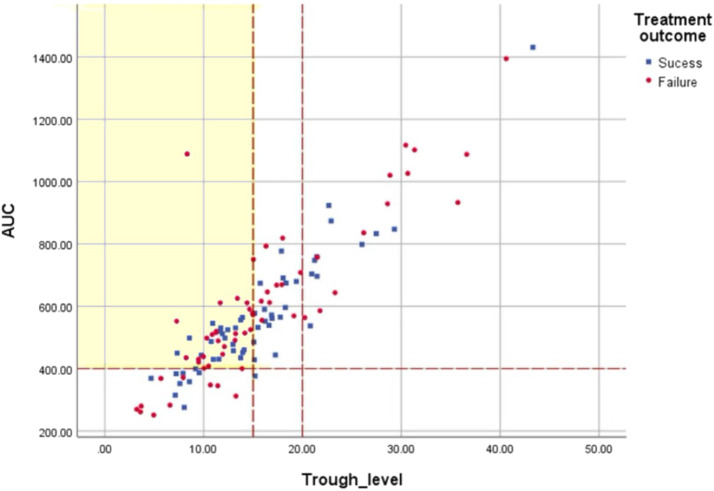
Correlation between vancomycin trough concentration and AUC
A positive correlation was identified between the trough concentration and AUC24 (Figure 2). The Pearson correlation coefficient was 0.849 (P < 0.001). However, 48 patients (40.7%) with trough levels below the currently recommended target of 15 mg/L achieved an AUC ≥400 mg· hr/L (see Supplemental Table 1 in the online version at doi:10.1016/j.curtheres.2022.100687). Only 14 patients (11.9%) achieved the target AUC of 400 to 600 mg· hr/L while within the target trough level of 15 to 20 mg/L.
Figure 2.
Scatter plot of vancomycin trough concentration and area under the concentration-time curve (AUC) (Pearson's correlation coefficient, r = 0.849; P < 0.001).
Clinical outcomes
A comparison of treatment success and failure is presented in Table 1. Overall, treatment failure was observed in 61 of the 118 patients (51.7%). The proportion of elderly patients (older than age 65 years) was higher in the treatment failure group, but the difference was not significant (45.6% vs 57.4%; P = 0.201). Patients with treatment failure had a higher men (SD) Charlson's WIC (2.04 [1.71] vs 3.15 [2.68]; P = 0.008), higher mean (SD) Pitt bacteremia scores (1.72 [1.81] vs 2.87 [2.51]; P = 0.005), and a longer mean (SD) duration of bacteremia (1.84 [2.04] vs 9.13 [6.60]; P < 0.001). Complicated infection (19.3% vs 86.9%; P < 0.001) and the development of acute kidney injury (26.3% vs 44.3%; P = 0.030) were more frequent in the treatment failure group than in the treatment success group.
Table 3 shows the risk factors associated with treatment failure identified using univariate and multivariate analyses. Comorbid solid organ tumors (adjusted OR [aOR] = 6.61; 95% CI, 1.19–36.81), cerebrovascular disease (aOR = 6.05; 95% CI, 1.17–31.23), complicated infection (aOR = 77.56; 95% CI, 16.74–359.37), and low body mass index (aOR = 0.94; 95% CI, 0.86–1.03) were independent risk factors for failure of vancomycin treatment. Of note, the severity of underlying disease and illness, represented by complicated infection and Pitt bacteremia score, had a greater effect on clinical outcomes than vancomycin exposure. Similarly, independent predictors of all-cause mortality included comorbid solid organ tumors (aOR = 17.29; 95% CI, 3.02–98.98), rheumatic disease (aOR = 12.35; 95% CI, 1.70–89.83), diabetes (aOR = 6.61; 95% CI, 1.53–28.49), high Pitt bacteremia score (aOR = 1.51; 95% CI, 1.09–2.09), complicated infection (aOR = 5.03; 95% CI, 1.61–21.85), and low body mass index (aOR = 0.84; 95% CI, 0.70–0.99). In addition, those who developed AKI during vancomycin treatment had a higher rate of all-cause mortality (aOR = 6.74; 95% CI, 1.82–24.99), although this was not found to be an independent predictor of treatment failure.
Table 3.
Risk factors for vancomycin treatment failure and 100-day mortality in patients with methicillin-resistant Staphylococcus aureus bacteremia determined by multivariate logistic regression analyses.
| Characteristic | Vancomycin treatment failure odds ratio |
100-d mortality outcome odds ratio |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|||||
| Odds ratio (95% CI) | P value | Adjusted odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Adjusted odds ratio (95% CI) | P value | |
| Age ≥65 y | 1.61 (0.78–3.32) | 0.202 | 2.06 (0.83–5.10) | 0.118 | ||||
| Female sex | 0.96 (0.46–2.03) | 0.921 | 1.02 (0.42–2.49) | 0.969 | ||||
| Body mass index | 0.94 (0.86–1.03) | 0.168 | 0.82 (0.70–0.96) | 0.012 | 0.89 (0.80–1.00) | 0.053 | 0.84 (0.70–0.99) | 0.04 |
| Underlying conditions | ||||||||
| Diabetes | 0.84 (0.39–1.79) | 0.644 | 2.29 (0.94–5.55) | 0.068 | 6.61 (1.53–28.49) | 0.011 | ||
| Cardiovascular disease | 1.70 (0.74–3.91) | 0.215 | 1.68 (0.66–4.31) | 0.277 | ||||
| Chronic liver disease | 0.93 (0.28–3.06) | 0.901 | 1.91 (0.53–6.93) | 0.325 | ||||
| Chronic lung disease | 1.70 (0.48–6.21) | 0.41 | 0.77 (0.16–3.80) | 0.747 | ||||
| Cerebrovascular disease | 1.90 (0.76–4.72) | 0.169 | 6.05 (1.17–31.23) | 0.032 | 1.15 (0.41–3.27) | 0.789 | ||
| Chronic kidney disease stage 3 to 5 | 1.26 (0.27–5.91) | 0.767 | 2.87 (0.60–13.73) | 0.187 | ||||
| Rheumatic disease | 1.45 (0.39–5.41) | 0.584 | 4.14 (1.10–15.63) | 0.036 | 12.35 (1.70–89.83) | 0.013 | ||
| Solid organ tumor | 3.56 (1.30–9.76) | 0.014 | 6.61 (1.19–36.81) | 0.031 | 5.71 (2.14–15.24) | < 0.001 | 17.29 (3.02–98.98) | 0.001 |
| Hematologic malignancy | 0.45 (0.08–2.55) | 0.367 | 0.70 (0.08–6.24) | 0.746 | ||||
| Immunosuppressive agents | 1.80 (0.57–5.74) | 0.32 | 4.47 (1.40–14.27) | 0.011 | ||||
| Septic shock | 2.12(1.01–4.42) | 0.046 | 3.80 (1.40–10.33) | 0.009 | ||||
| Pitt bacteremia score | 1.25 (1.04–1.50) | 0.018 | 1.32 (0.98–1.78) | 0.072 | 1.44 (1.16–1.79) | 0.001 | 1.51 (1.09–2.09) | 0.012 |
| Complicated infection | 27.71 (10.27–74.76) | < 0.001 | 77.56 (16.74–359.37) | < 0.001 | 4.79 (1.66–13.78) | 0.004 | 5.03 (1.16–21.85) | 0.031 |
| Development of acute kidney injury | 2.22 (1.02–4.83) | 0.044 | 8.14 (3.04–21.84) | < 0.001 | 6.74 (1.82–24.99) | 0.004 | ||
| Area under the concentration-time curve cut-off <373.5 mg· hr/L | 2.04 (0.65–6.38) | 0.221 | 0.87 (0.23-3.35) | 0.839 | ||||
AKI
A comparison between patients with and without nephrotoxicity is presented in Table 2. Elderly patients (aged 65 years and older) were more prevalent in the group with nephrotoxicity (64.3% vs 44.7% in the group without nephrotoxicity; P = 0.042), whereas sex distribution and body mass index were not statistically different between the 2 groups. Patients who experienced nephrotoxicity had higher Charlson's WIC and Pitts bacteremia scores and were more likely to have received nephrotoxic agents and have underlying chronic kidney disease and complicated infection. All 5 patients with endocarditis as the primary infection experienced nephrotoxicity (11.9% vs 0.0% in the group without nephrotoxicity; P = 0.005).
Predictors of vancomycin-induced nephrotoxicity are shown in Table 4. Age older than 65 years (aOR = 3.59; 95% CI, 1.17–10.97), concomitant use of nephrotoxins (aOR = 12.88; 95% CI, 1.25–133.15), underlying chronic liver disease (aOR = 7.52; 95% CI, 1.37–41.37), chronic kidney disease stage 3 or greater (aOR = 29.27; 95% CI, 1.68–510.28), and a high Pitts bacteremia score (aOR = 1.57; 95% CI, 1.18–2.10) were putative predictors of nephrotoxicity. Furthermore, an AUC threshold ≥615.0 mg· hr/L according to ROC curve analysis was associated with an increased risk of nephrotoxicity (aOR = 5.24; 95% CI, 1.88–14.65).
Table 4.
Risk factors for vancomycin-induced nephrotoxicity in patients with methicillin-resistant Staphylococcus aureus bacteremia determined by multivariate logistic regression analyses.
| Characteristic | Nephrotoxicity odds ratio |
|||
|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
|||
| Odds ratio (95% CI) | P value | Adjusted odds ratio (95% CI) | P value | |
| Age ≥65 y | 2.22 (1.02–4.83) | 0.044 | 3.59 (1.17–10.97) | 0.025 |
| Male sex | 0.99 (0.46–2.17) | 0.995 | ||
| Body mass index | 0.97 (0.87–1.07) | 0.556 | ||
| Charlson's weighted index of comorbidity | 1.20 (1.02–1.41) | 0.032 | ||
| Underlying conditions | ||||
| Diabetes | 1.26 (0.57–2.75) | 0.57 | ||
| Cardiovascular disease | 2.08 (0.90–4.82) | 0.086 | ||
| Chronic liver disease | 2.84 (0.84–9.59) | 0.093 | 7.52 (1.37–41.37) | 0.020 |
| Chronic lung disease | 0.65 (0.16–2.61) | 0.547 | ||
| Cerebrovascular disease | 1.57 (0.64–3.86) | 0.325 | ||
| Chronic kidney disease stage 3 to 5 | 12.50 (1.45–107.74) | 0.022 | 29.27 (1.68–510.28) | 0.021 |
| Rheumatic disease | 1.23 (0.36–4.62) | 0.761 | ||
| Solid organ tumor | 2.13 (0.86–5.30) | 0.103 | ||
| Hematologic malignancy | 0.35 (0.04–3.07) | 0.341 | ||
| Immunosuppressive agents | 1.42 (0.46–4.40) | 0.547 | ||
| Septic shock | 1.71 (0.79–3.69) | 0.17 | ||
| Pitt bacteremia score | 1.26 (1.05–1.52) | 0.013 | 1.57 (1.18–2.10) | 0.002 |
| Complicated infection | 2.61 (1.18–5.79) | 0.018 | ||
| Bacteremia duration, d | 1.05 (0.99–1.11) | 0.135 | ||
| Concomitant use of nephrotoxins | 13.67 (1.76–106.22) | 0.012 | 12.88 (1.25–133.15) | 0.032 |
| Area under the concentration-time curve cut-off ≥615.0 mg· hr/L | 3.01 (1.29–7.02) | 0.011 | 5.24 (1.88–14.65) | 0.002 |
Identification of AUC thresholds for treatment failure and nephrotoxicity
The threshold for dichotomizing the AUC data was determined using CART and ROC analysis. The predictive performance of the CART-and ROC-derived AUC thresholds for nephrotoxicity and treatment failure are listed in Supplemental Tables 2 and 3, respectively (available in the online version at doi:10.1016/j.curtheres.2022.100687). A ROC-derived AUC nephrotoxicity threshold of 615.0 mg· hr/L had the highest positive predictive value and highest area under the ROC curve and was therefore incorporated into the multivariate analysis (see Supplemental Table 2 in the online version at doi:10.1016/j.curtheres.2022.100687). According to the multivariate analysis, an AUC threshold ≥615.0 mg· hr/L according to ROC curve analysis was associated with an increased risk of nephrotoxicity (aOR = 5.24; 95% CI, 1.88–14.65) (Table 4).
In contrast, it was impossible to discriminate AUC thresholds for treatment failure because the means of the AUC for the treatment success and failure groups were not significantly different (P = 0.302), and neither the CART nor the ROC-derived AUC thresholds were significantly predictive (see Supplemental Table 3 in the online version at doi:10.1016/j.curtheres.2022.100687).
Discussion
Recent studies have shown that AUC-based monitoring offers the opportunity to optimize treatment efficacy and minimize nephrotoxicity by avoiding unnecessary exposure to vancomycin.4,19 With the paradigm shift from the use of trough concentration to AUC in vancomycin TDM, we aimed to evaluate the association between vancomycin exposure and treatment outcome with a focus on the establishment of treatment failure and toxicity thresholds. Several important conclusions can be drawn from this study involving hospitalized adult patients with MRSA bacteremia.
In most patients, the optimal AUC/MIC did not occur within the recommended trough target range. Similar to previous studies, a substantial number of patients achieved an AUC ≥400 mg· hr/L at trough levels below the guideline recommendations (≤15 mg/L).4,5,20 In these patients, the vancomycin dose was increased to attain the trough target, resulting in unnecessarily higher exposures and an increased risk of AKI. This discordance is not surprising, considering the definitions of trough concentration and AUC. The AUC is the cumulative exposure for a defined time period, whereas the trough concentration is reflective of only a single exposure point at the end of the dosing interval.4,19 Maintaining trough concentrations of 15 to 20 mg/L equates to an AUC between 360 mg· hr/L (15 mg/L × 24 hours) and 480 mg· hr/L (20 mg/L × 24 hours), ensuring an AUC ≥400 mg· hr/L in most cases. However, there is high interpatient variability in the peak concentrations and upper range of AUC values associated with a given trough value. Therefore, AUC-guided vancomycin dosing should be considered instead of trough-guided monitoring to ensure an accurate and safe dosage regimen.
Overall, treatment failure and nephrotoxicity rates of 51.7% and 35.6%, respectively, were observed. The severity of acute illness and comorbidities were clinically relevant risk factors for treatment failure. In particular, patients with solid organ malignancies were more likely to experience treatment failure (12.1% vs 28.3%; P = 0.028). Vancomycin treatment success was predominantly observed in patients with low-risk sources of infection, such as intravenous catheter-related bloodstream infections (37.9% vs 18.3%; P = 0.018), whereas clinical failure was mostly observed in high-inoculum infections, including endocarditis and central nervous system infections. This is not entirely unexpected because persistent bacteremia and consequent metastatic infection are commonly associated with high-inoculum infections.21,22 Nevertheless, patients from all categories of infection source experienced treatment failure and mortality, implicating the significance of comorbidity or presence of prostheses.21,23 In our study, the highest proportion of patients with MRSA bacteremia who experienced treatment failure was of those with bone and joint infection (28.3%), most of whom had prosthetic materials implanted. Because our hospital is among the largest trauma centers in Korea, the majority of patients who possess prostheses visit us, which explains the higher number of cases of complicated infections and treatment failures reported in this study than in previous studies.
According to previous clinical studies, a low vancomycin AUC/MIC is an independent risk factor for treatment failure in MRSA bacteremia.13,21,24,25 Song et al13 reported that an AUC/MIC <392.7 (by BMD) and <397.2 (by Etest) was associated with treatment failure, whereas Kullar et al26 showed that an AUC/MIC <421 was associated with significantly higher rates of treatment failure. In 2014, Lodise et al12 demonstrated that AUC/MIC thresholds ≥650 (by BMD) and ≥320 (by Etest) were associated with a lower probability of treatment failure, with risk ratios approximately 0.5 for all AUC/MIC exposure thresholds.12 This inconsistency creates uncertainty regarding the predictive value of the AUC/MIC parameter. Thus, we attempted to discriminate AUC/MIC thresholds for treatment failure using CART and ROC analyses. However, both CART- and ROC-derived AUC thresholds for treatment failure were not significant predictive values (see Supplemental Table 3 available in the online version at doi:10.1016/j.curtheres.2022.100687). Instead, underlying comorbid condition and complicated infections were significantly associated with an increased risk of treatment failure, indicating that higher vancomycin exposures do not provide a clinical benefit. Although clinical efficacy was not associated with vancomycin exposure, the risk of AKI increased with higher AUC/MIC. This finding is consistent with recent reports.9,10 A prospective, multicenter observational study by Lodise et al10 showed that no difference was observed in treatment failure between prespecified vancomycin exposure groups (AUC/MICBMD ≥650 and AUC/MICEtest ≥320), whereas higher rates of AKI were found in the groups with a higher AUC/MIC on day 2. They also illustrated that patients with an AUC <515 mg·hr/L on day 2 experienced the best overall outcome (ie, fewer instances of AKI and treatment failure) based on a Desirability of Outcome Ranking (DOOR) risk-benefit analysis.
According to our risk factor analysis for all-cause mortality in MRSA bacteremia, the severity of illness and comorbidity, as well as the development of AKI, confers a higher risk of mortality (aOR = 6.74; 95% CI, 1.82–24.99), indicating the importance of avoiding unnecessarily high vancomycin exposure. Thus, defining the upper limit of the therapeutic AUC is crucial for achieving the best clinical outcome. In the current analysis, ROC-derived thresholds ≥615.0 mg· hr/L for the nephrotoxicity AUC were significant and had the most predictive value, maximizing the positive predictive value. This cutoff value was identified as an independent predictor of AKI using multivariate logistic regression analysis (aOR = 5.92; 95% CI, 2.23–15.70) (Table 3), and only the AUC remained significant (aOR = 1.005; 95% CI, 1.002–1.007) despite the observed correlation between trough concentration and AUC (data not shown in Table 3). In this study, the nephrotoxicity threshold was lower than the previously predicted cutoff between 648 and 1300 mg· hr/L.14,15,24 This disparity may be explained by differences in patient populations or the definitions of nephrotoxicity. The proportion of comorbid chronic kidney disease patients differed amongst previous studies, and various exclusion criteria were applied. Chavada et al5 and the present study only excluded dialysis patients, whereas Zasowski et al14 excluded patients with a baseline serum creatinine value ≥2 mg/dL. This difference could lead to a wide range of AUC thresholds for nephrotoxicity. Moreover, the criteria used for defining AKI were adopted from Acute Kidney Injury Network and the Kidney Disease: Improving Global Outcomes, which define AKI as a serum creatinine level of 0.3 mg/L or a 50% increase from baseline on 2 or more consecutive measurements, which are more sensitive parameters than those reported in previous studies.5,11,14,24 It is logical that more sensitive definitions of AKI would result in lower AUC nephrotoxicity thresholds.
This study has some limitations that must be considered. First, there are limitations inherent to the study design, including selection bias. Because this was a study of adult patients with MRSA bacteremia who were not on dialysis, the observed findings are not applicable to other populations. Additionally, the enrollment criteria of including patients with blood culture monitored every 3 to 5 days could confound the assessment of duration of the bacteremia. Second, because the first author and the single pharmacist mainly inspected all the patients’ records several times and conducted an AUC prediction, assessment of observer variability was not performed. Third, the AUC estimation was performed at a steady state using a single trough concentration. In fact, it is difficult to determine steady states in clinical practice, and we were not absolutely certain that all patients were truly in a steady state. Our institutional dosing guidelines recommend 15 to 20 mg/kg every 12 hours in adult patients with normal renal function, and to monitor the vancomycin concentration before the fourth or fifth dose in all patients. Thus, by not accounting for individuals’ different pharmacokinetic parameters, our AUC estimation could cause delay in achieving desired concentrations. Moreover, although a reliable estimation of the daily AUC could be obtained using a Bayesian software program with trough-only pharmacokinetic sampling, a Bayesian prior embedded with richly sampled vancomycin data, mainly obtained from western population might be inadequate for Korean population. Because this study is a retrospective study, a second level was unavailable from our study. Although using single drug level is more convenient and less costly, it may not be as accurate as a 2-level approach, particularly in patients with very altered pharmacokinetic parameters.27 Lastly, the sample size was small; therefore, other possible risk factors for clinical failure in MRSA bacteremia may have appeared to be insignificant.
This study was strengthened by the use of a Bayesian approach to estimate an individual's vancomycin exposure profile with limited vancomycin blood concentration data. AUC determination originally required multiple pharmacokinetic sampling to calculate AUC by linear trapezoidal method. An equation-based approach should involve at least 2 times of pharmacokinetic sampling, whereas Bayesian approach could estimate AUC with collection of 1 or 2 concentrations.27 Recent analysis validating Bayesian dose-optimizing software demonstrated that the addition of a second level to the trough could improve the accuracy and bias in some software, especially in critically ill patients.28 Al-sulaiti et al27 also demonstrated that peak-trough based TDM could improve vancomycin-associated cure. Although it is preferred to estimate the Bayesian AUC using 2-point concentrations, trough-only pharmacokinetic sampling can be utilized to generate reliable estimates of AUC with the help of population pharmacokinetic databases supplied by the software as Bayesian prior. Neely et al4 and Pai et al19 demonstrated that Bayesian AUC estimated from trough-only data was associated with 97% accurate AUC estimation (93%–102%; P = 0.23), when using multiple concentration-time profile as Bayesian prior. However, there were limited inclusion of special populations in previously mentioned studies, and it is unclear whether this single point Bayesian AUC estimates could be applicable to general population. In addition, using a single nontrough value during beta-elimination phase with Bayesian software produces similar estimates with trough-only values.28,29 Thus, our study could overcome the problem with inaccuracy of sampling time or single point estimates of AUC by using a Bayesian dose-optimizing software program, to some extent. Furthermore, to determine the most predictive AUC cutoff for clinical failure and nephrotoxicity, both CART and ROC methods were attempted, and these candidate AUC values were evaluated for their predictive performance, which is unique compared with previous studies.
Conclusions
The results of this study demonstrated a modest ability of the vancomycin AUC to predict clinical outcomes. Rather than vancomycin exposure, comorbid diseases, severity of acute illness, and lower body mass index were independent risk factors for clinical failure. However, given that a wide variety of individuals’ clinical situations, comorbid diseases, and concomitant therapies in real world, a 1-size-fits-all approach to drug dose delivery would be less accurate, and no longer reasonable. Therefore, more specific and individualized AUC targets should be considered, but require further study and confirmation before implementation. A higher vancomycin exposure was associated with an increased risk of AKI. We defined the upper limit of the vancomycin therapeutic range, with a vancomycin AUC ≥615.0 mg· hr/L, as associated with a nearly 5-fold increase in nephrotoxicity. Further studies are needed to define the AUC therapeutic range to maximize efficacy and minimize the likelihood of toxicity.
Conflicts of Interest
The authors have indicated that there is no conflict of interest regarding the content of this article.
Acknowledgments
This work was supported by the intramural research fund of Ajou University Medical Center at 2018. The authors thank Editage (www.editage.co.kr) for editing and reviewing this manuscript for English language.
Y. Kim, J. Heo, and E. Kim designed the study. Y. Kim, H.-J. Chun, and J. Heo obtained and analyzed the data. H.-J. Chun performed the pharmacometric analysis. Y. Kim wrote the original draft. Y. Kim, J. Heo, and E. Kim revised the manuscript. Y. Kim, E. Kim, J. Yoo, and Y. Choi reviewed the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.curtheres.2022.100687.
Appendix. Supplementary materials
References
- 1.Rodvold KA. 60 Plus Years Later and We Are Still Trying to Learn How to Dose Vancomycin. Clinical Infectious Diseases. 2019;70:1546–1549. doi: 10.1093/cid/ciz467. [DOI] [PubMed] [Google Scholar]
- 2.Rybak MJ, Rotschafer JC, Rodvold KA. Vancomycin: over 50 years later and still a work in progress. Pharmacotherapy. 2013;33:1253–1255. doi: 10.1002/phar.1382. [DOI] [PubMed] [Google Scholar]
- 3.Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49:325–327. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 4.Neely MN, Youn G, Jones B, et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014;58:309–316. doi: 10.1128/AAC.01653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavada R, Ghosh N, Sandaradura I, Maley M, Van Hal SJ. Establishment of an AUC0-24 Threshold for Nephrotoxicity Is a Step towards Individualized Vancomycin Dosing for Methicillin-Resistant Staphylococcus aureus Bacteremia. Antimicrob Agents Chemother. 2017:61. doi: 10.1128/AAC.02535-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tongsai S, Koomanachai P. The safety and efficacy of high versus low vancomycin trough levels in the treatment of patients with infections caused by methicillin-resistant Staphylococcus aureus: a meta-analysis. BMC Res Notes. 2016;9:455. doi: 10.1186/s13104-016-2252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsutsuura M, Moriyama H, Kojima N, et al. The monitoring of vancomycin: a systematic review and meta-analyses of area under the concentration-time curve-guided dosing and trough-guided dosing. BMC Infect Dis. 2021;21:153. doi: 10.1186/s12879-021-05858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77:835–864. doi: 10.1093/ajhp/zxaa036. [DOI] [PubMed] [Google Scholar]
- 9.Dalton BR, Rajakumar I, Langevin A, et al. Vancomycin area under the curve to minimum inhibitory concentration ratio predicting clinical outcome: a systematic review and meta-analysis with pooled sensitivity and specificity. Clin Microbiol Infect. 2020;26:436–446. doi: 10.1016/j.cmi.2019.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The Emperor's New Clothes: PRospective Observational Evaluation of the Association Between Initial VancomycIn Exposure and Failure Rates Among ADult HospitalizEd Patients With Methicillin-resistant Staphylococcus aureus Bloodstream Infections (PROVIDE) Clin Infect Dis. 2020;70:1536–1545. doi: 10.1093/cid/ciz460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung Y, Song KH, Cho J, et al. Area under the concentration-time curve to minimum inhibitory concentration ratio as a predictor of vancomycin treatment outcome in methicillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 2014;43:179–183. doi: 10.1016/j.ijantimicag.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Lodise TP, Drusano GL, Zasowski E, et al. Vancomycin exposure in patients with methicillin-resistant Staphylococcus aureus bloodstream infections: how much is enough? Clin Infect Dis. 2014;59:666–675. doi: 10.1093/cid/ciu398. [DOI] [PubMed] [Google Scholar]
- 13.Song KH, Kim HB, Kim HS, et al. Impact of area under the concentration-time curve to minimum inhibitory concentration ratio on vancomycin treatment outcomes in methicillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 2015;46:689–695. doi: 10.1016/j.ijantimicag.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Zasowski EJ, Murray KP, Trinh TD, et al. Identification of Vancomycin Exposure-Toxicity Thresholds in Hospitalized Patients Receiving Intravenous Vancomycin. Antimicrob Agents Chemother. 2018:62. doi: 10.1128/AAC.01684-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009;49:507–514. doi: 10.1086/600884. [DOI] [PubMed] [Google Scholar]
- 16.Aljefri DM, Avedissian SN, Rhodes NJ, Postelnick MJ, Nguyen K, Scheetz MH. Vancomycin Area Under the Curve and Acute Kidney Injury: A Meta-analysis. Clin Infect Dis. 2019;69:1881–1887. doi: 10.1093/cid/ciz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diekema DJ, Pfaller MA, Shortridge D, Zervos M, Jones RN. Twenty-Year Trends in Antimicrobial Susceptibilities Among Staphylococcus aureus From the SENTRY Antimicrobial Surveillance Program. Open Forum Infect Dis. 2019;6:S47–S53. doi: 10.1093/ofid/ofy270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50–57. doi: 10.1016/j.addr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Sohn YM, Park HJ, Chung JE, et al. Low Correlation Between Vancomycin Area Under the Curve Over 24 Hours to the MIC (AUC/MIC ratio) and the Trough Concentration at Steady State in Methicillin-Resistant Staphylococcus Aureus Pneumonia. Journal of Korean Society of Health-System Pharmacists. 2020;37:408–416. [Google Scholar]
- 21.Ghosh N, Chavada R, Maley M, van Hal SJ. Impact of source of infection and vancomycin AUC0-24/MICBMD targets on treatment failure in patients with methicillin-resistant Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2014;20:O1098–O1105. doi: 10.1111/1469-0691.12695. [DOI] [PubMed] [Google Scholar]
- 22.Soriano A, Marco F, Martinez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 23.Hassoun A, Linden PK, Friedman B. Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit Care. 2017;21:211. doi: 10.1186/s13054-017-1801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogle BT, Steele JM, Seabury RW, Dang UJ, Kufel WD. Implementation of a two-point pharmacokinetic AUC-based vancomycin therapeutic drug monitoring approach in patients with methicillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 2018;52:805–810. doi: 10.1016/j.ijantimicag.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 25.Moise PA, Forrest A, Bhavnani SM, Birmingham MC, Schentag JJ. Area under the inhibitory curve and a pneumonia scoring system for predicting outcomes of vancomycin therapy for respiratory infections by Staphylococcus aureus. Am J Health Syst Pharm. 2000;57(Suppl 2):S4–S9. doi: 10.1093/ajhp/57.suppl_2.S4. [DOI] [PubMed] [Google Scholar]
- 26.Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52:975–981. doi: 10.1093/cid/cir124. [DOI] [PubMed] [Google Scholar]
- 27.Al-Sulaiti FK, Nader AM, Saad MO, et al. Clinical and Pharmacokinetic Outcomes of Peak-Trough-Based Versus Trough-Based Vancomycin Therapeutic Drug Monitoring Approaches: A Pragmatic Randomized Controlled Trial. Eur J Drug Metab Pharmacokinet. 2019;44:639–652. doi: 10.1007/s13318-019-00551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner RB, Kojiro K, Shephard EA, et al. Review and Validation of Bayesian Dose-Optimizing Software and Equations for Calculation of the Vancomycin Area Under the Curve in Critically Ill Patients. Pharmacotherapy. 2018;38:1174–1183. doi: 10.1002/phar.2191. [DOI] [PubMed] [Google Scholar]
- 29.Neely MN, Kato L, Youn G, et al. Prospective Trial on the Use of Trough Concentration versus Area under the Curve To Determine Therapeutic Vancomycin Dosing. Antimicrob Agents Chemother. 2018:62. doi: 10.1128/AAC.02042-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.