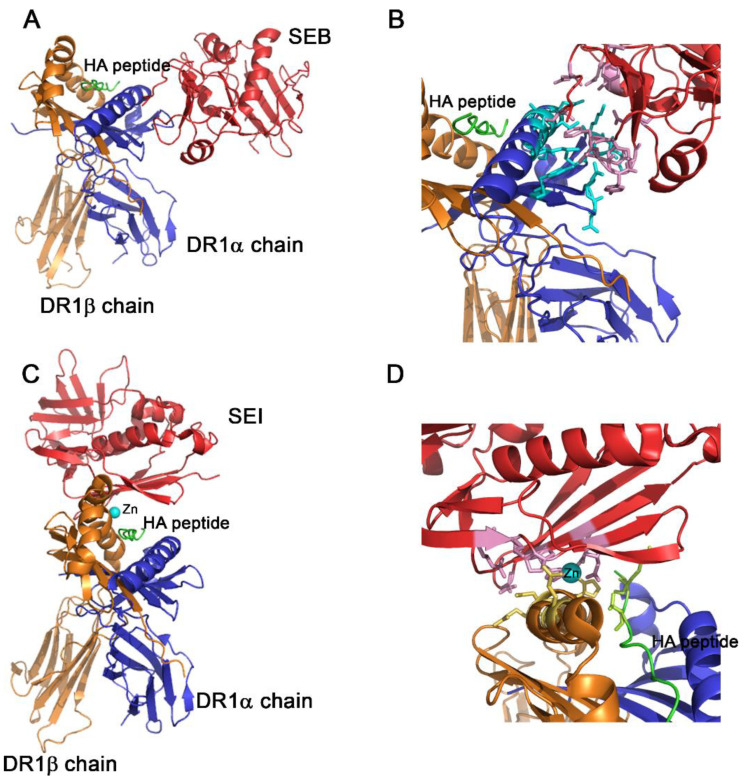
Figure 2.
Structure of the SAg-HA-HLA DR1 complex. (A) Ribbon diagram of SEB-HA-HLA DR1 complex. (B) The interface of the interaction is shown in detail. SEB compromises residues in positions 43, 44, 45, 46, 47, 67, 89, 92, 94, 95, 115 and 209. HLA DR1α chain, involves the residues: 13, 17, 18, 36, 37, 39, 57, 60, 61, 63, 67 and 68. Non-contacts are found between the peptide and SEB or SEB with the DR1β chain. (C) Ribbon diagram of the SEI-HA-HLA DR1 complex. (D) The interface of the interaction is shown in detail. The interaction is coordinated by Zn2+. This metal ion interacts with His81 of the DR1β chain and His169, His207 and Asp209 of SEI. SEI compromises residues in positions 98, 100, 105 and 211 to contact the residues 307 and 309 of the hemagglutinin (HA). No contacts are found between SEI and the DR1α chain. In all panels, the superantigen is colored red; the HLAD1α chain, blue; and the DR1β chain, orange. Zn2+ is represented as a sphere in cyan and the HA peptide, green. The residues conforming the interaction surface are represented as balls and sticks and colored pink (SEB or SEI); cyan, HLAD1α chain; yellow, DR1β chain; and light green, HA peptide. The figures were performed using PyMOL and the analysis of the structures was carried out using the CCP4i suite program.