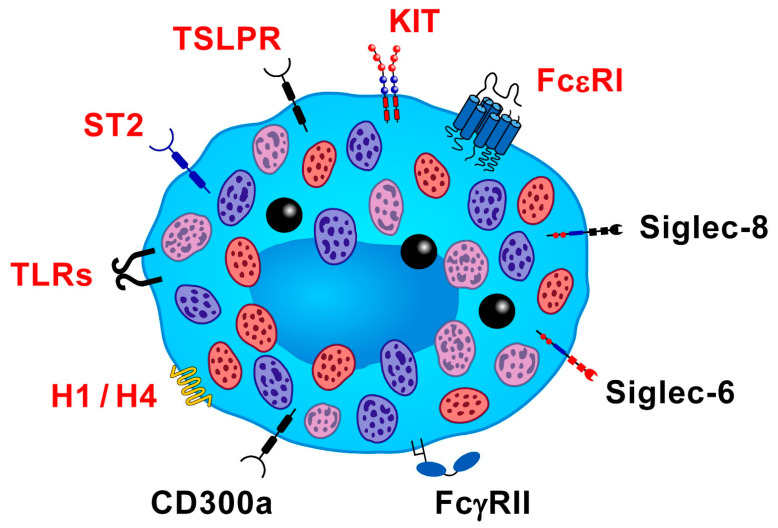
Figure 1.
Schematic representation of activating (in red) and inhibitory (in black) receptors on human lung mast cells. A complete (αβγ2), high-affinity receptor for IgE (FcεRI) is expressed by human mast cells and basophils [31]. Antigens, superantigens, and anti-IgE lead to mast cell activation and mediator release through the aggregation of FcεRI bound to IgE [31,32,33]. Mast cells play a role in allergic disorders by releasing preformed (e.g., histamine, tryptase) and de novo synthesized mediators (e.g., cysteinyl leukotriene C4 (LTC4), prostaglandin D2 (PGD2)) and several cytokines and chemokines [3,19]. SCF, which activates the KIT receptor (CD117), highly expressed by mast cells throughout their development, is the most important factor regulating these cells [34,35]. SCF is released by a plethora of cells, including fibroblasts, endothelial cells, and mast cells themselves [36,37]. Thymic stromal lymphopoietin (TSLP) is expressed by airway epithelial cells [38], keratinocytes [39], immune and structural cells [40] and acts as an alarmin. TSLP activates the heterodimeric receptor comprising IL-7Rα and TSLP receptor (TSLPR) on mast cells and other immune cells [41,42,43]. TSLPR mRNA, but not IL-7Rα mRNA, is expressed by human mast cells [42,44]. TSLP, under certain experimental conditions [42], can induce the release of chemokines and cytokines from mast cells [45]. IL-33 is released by damaged epithelial and endothelial cells [46,47] and activates mast cells [48,49]. IL-33 engages the heterodimeric receptor, ST2-IL-1RAcP, on human mast cells [50,51,52] and induces cytokine [50,53,54,55,56] and chemokine release [57]. IL-33 and superantigenic activation cause the release of angiogenic and lymphangiogenic factors from human lung mast cells (HLMCs) [58]. Different mast cell subsets display distinct toll-like receptors (TLRs) [59]. Activation of TLR2, -3, -4, -6, -7, and -9 induce cytokine release from human mast cells [60,61,62]. FcεRI cross-linking amplifies TLR-induced cytokine released from human mast cells [63]. Histamine is preformed in cytoplasmic granules of human mast cells (≅3 pg/cell) and basophils (≅1 pg/cell) [64,65]. Human mast cells express the histamine H1 and H4 receptors [66]. Histamine induces exocytosis and IL-6 production from human lung macrophages by activating H1 receptors [67]. High concentrations of certain H1-antihistamines can inhibit mediator release from human FcεRI+ cells [68,69]. H4R is expressed by human mast cells, and its activation can modulate the function of these cells [66]. Mast cells display several inhibitory receptors [70,71], such as CD300a and CD200R [72,73]. Coaggregation of CD300a and FcεRI with a bispecific antibody inhibits IgE-mediated tryptase and IL-4 release from human mast cells [74] and IgE-mediated anaphylaxis in preclinical asthma models [75]. Siglecs are inhibitory receptors expressed on immune cells [76]. Siglec-8 is expressed on murine and human mast cells [77,78] and on mast cell lines [76,77,79]. Siglec-8 monoclonal antibody (mAb) diminishes FcεRI-mediated histamine and PGD2 release from mast cells [80]. Siglec-8 is also present on eosinophils and basophils [81,82]. Siglec-6 is selectively expressed by mast cells [76], and a mAb cross-linking this receptor (AK006) potently inhibits IgE-mediated human mast cell activation. Siglec-6 interacts with KIT/CD117 and inhibits SCF-mediated mast cell activation (Korver, Schanin, 10th EMBRN meeting, July 11–12, 2022). Human mast cells express the activating FcγRIIA and FcγRI induced by IFN-γ [83]. A human IgG-IgE Fc fusion protein co-crosslinks FcεRI and FcγRII receptors and inhibits histamine release from human basophils and HLMCs [84,85,86]. A dual-targeting tandem IgE-IgG Fc domain inhibits mast cell degranulation [87].