Abstract
Zinc (Zn) is an essential micronutrient for rice, but it is toxic at a high concentration, especially in acid soils. It is yet unknown which genes regulate Zn tolerance in rice. In the present study, a genome-wide association study (GWAS) was performed for Zn tolerance in rice at the seedling stage within a rice core collection, named Ting’s core collection, which showed extensive phenotypic variations in Zn toxicity with high-density single-nucleotide polymorphisms (SNPs). A total of 7 and 19 quantitative trait loci (QTL) were detected using root elongation (RE) and relative root elongation (RRE) under high Zn toxicity, respectively. Among them, 24 QTL were novel, and qRRE15 was located in the same region where 3 QTL were reported previously. In addition, qRE4 and qRRE9 were identical. Furthermore, we found eight candidate genes that are involved in abiotic and biotic stress, immunity, cell expansion, and phosphate transport in the loci of qRRE8, qRRE9, and qRRE15. Moreover, four candidate genes, i.e., Os01g0200700, Os06g0621900, Os06g0493600, and Os06g0622700, were verified correlating to Zn tolerance in rice by quantitative real time-PCR (qRT-PCR). Taken together, these results provide significant insight into the genetic basis for Zn toxicity tolerance and tolerant germplasm for developing rice tolerance to Zn toxicity and improving rice production in Zn-contaminated soils.
Keywords: rice, Ting’s core collection, Zn toxicity, GWAS, QTL
1. Introduction
Zinc (Zn) is an essential micronutrient for rice growth and development, as it is involved in numerous physiological and biochemical processes [1]. However, human activities, such as mining, sewage sludge treatment in agricultural soils, and anthropogenic Zn inputs in urban and peri-urban soils, cause Zn contamination [1,2]. Excess Zn in soil is toxic to rice and results in growth inhibition, as well as yield reduction, especially in acidic soils [1]. Therefore, improving Zn tolerance is significantly meaningful to rice production.
Deciphering the genetic mechanism for Zn tolerance in rice is the premise of improving rice Zn tolerance. Plants have developed resistant mechanisms responding to Zn toxicity, which include translocation from root to shoot, sequestration into vacuoles, and homeostasis with iron [3,4,5,6,7,8,9,10,11]. Moreover, rice show a wide range of natural variations and differ markedly in their susceptibility to Zn toxicity [12,13,14,15,16]. Plant physiologists and breeders have been focusing on revealing the genetic mechanism of Zn tolerance in rice; however, more studies still need to be performed, in the future, due to the complexity of the genetic mechanism for Zn tolerance in rice.
A total of 74 QTL for Zn tolerance in rice have been reported in previous research via linkage mapping and GWAS [12,14,15,16]. These QTL are associated with some easily measurable traits, such as leaf discoloring index, shoot height, root length, and shoot and root fresh weight and dry weight under Zn toxicity conditions at the seedling stage. For instance, a major QTL, qZNT-1 on chromosome 1, shows an effect with an LOD value of 6.0 and explains 21.9% of the total phenotypic variation using leaf discoloring index [12]. qZnRL11 from chromosome 11 [15] is co-located with the QTL for relative shoot dry weight and relative total dry weight [14]. qSFW3b for shoot fresh weight and qSDW3b for shoot dry weight [16] are co-located with qZNT-3 [12]. We summarized QTL and found that rice Zn tolerance genes were not cloned, and this was, for the greater part, due to the difficulty of evaluating Zn tolerance in rice.
Phenotyping is a prerequisite for evaluating heavy metal tolerance in plants. In Arabidopsis, root length growing under heavy metal treatment is frequently used to evaluate heavy metal tolerance, and some tolerant and sensitive accessions were identified by using root length under heavy metal toxicity. Furthermore, well-known genes such as FRO2, GSNOR, and SRF3 were located through GWAS [17,18,19,20].
A variety of plants from highly contrasting environments showed extensive natural variation and increased the probability of identifying causal variants [20]. Rice landraces from Ting’s core collection were collected from different environments [21], which showed abundant genetic diversity and significantly different adaptations to abiotic stress, especially cadmium toxicity (data not provided) and aluminum toxicity [22,23]. Ting’s core collection was used for GWAS on rice agronomic traits [24], aluminum tolerance [22,23], and sheath blight resistance [25]. Therefore, Ting’s core collection might be an appropriate population for GWAS on rice Zn tolerance.
In the present study, we performed a GWAS with more than 4.3 million high-quality SNPs by using root elongation and relative root elongation (root elongation in high Zn conditions/root elongation in control conditions) under high Zn toxicity at the seedling stage within Ting’s core collection. This study aims to (1) identify the candidate genes corresponding to Zn toxicity and (2) evaluate the landraces with strong Zn tolerance in Ting’s core collection. This study may provide information for improving rice Zn tolerance in potentially Zn-contaminated soils.
2. Results
2.1. Identification of Phenotypic Variations for Zn Tolerance within Ting’s Core Collection
We made use of Ting’s core collection and assessed the phenotypic variations in root elongation under Zn toxicity. The root elongation (RE) and relative root elongation (RRE, root elongation in high Zn/root elongation in control) of Ting’s core collection were measured before and after Zn exposure. Extensive phenotypic variations in root elongation in response to Zn toxicity were observed (Figure 1a,c; Table S1). The average root elongation was 43.54 mm with a range from 16.71 mm to 56.12 mm under control conditions and 20.16 mm with a range of 8.25–34.39 mm under high Zn conditions. The average relative root elongation was 46.42%, ranging from 21.86% to 78.19% (Table 1). On the basis of the value of relative root elongation, one variety, named You zhan hong, which was collected from South China, was inhibited under high Zn toxicity (relative root elongation was 21.86%), while one variety, named Nagabo, from Taiwan of China, showed the highest Zn tolerance (relative root elongation was 78.19%) (Table S1).
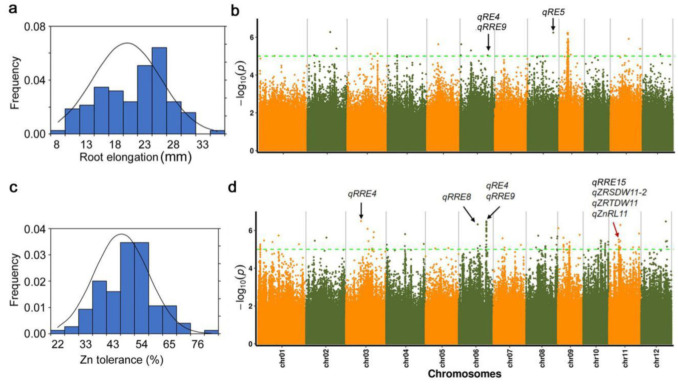
Figure 1.
GWAS of Zn tolerance by using EMMAX model. (a) Distribution of root elongation of Ting’s core collection growing under Zn toxicity (120 μM); (b) Manhattan plot of GWAS using root elongation under Zn toxicity (120 μM); (c) distribution of relative root elongation of Ting’s core collection growing under Zn toxicity (120 μM); and (d) Manhattan plot of GWAS using relative root elongation. Chromosomes are depicted in different colors. The horizontal green dashed line indicates the significance threshold (−log10(mBF) = 5.02). The black arrows represent the novel loci associated with Zn tolerance detected in the present study. The red arrow represents the loci, which were reported previously.
Table 1.
Performance of Ting’s core collection under control conditions and Zn toxicity.
| Trait | Range | Mean ± SD |
|---|---|---|
| Root elongation (RE, control) | 16.71–56.12 (mm) | 43.54 ± 8.95 (mm) |
| Root elongation (RE, under 120 μM Zn) | 8.25–34.39 (mm) | 20.16 ± 5.91 (mm) |
| Relative root elongation (RRE, %) | 21.86–78.19 (%) | 46.42 ± 10.48 (%) |
2.2. GWAS for Root Elongation under High Zn Toxicity within Ting’s Core Collection
The data of genetic analysis referenced from our previous studies, in which genetic analysis included the linkage disequilibrium (LD) decay distance, population structure, and kinship value of Ting’s core collection required for GWAS, were uncovered well.
A total of 4,395,003 high-quality SNPs were used to perform GWAS. In the present study, seven novel QTL on chromosomes 2, 6, 8, 9, and 11 were identified as significantly (p < 0.94 × 10−5) associated with root elongation under high Zn toxicity by using the EMMAX model (Table 2). Among them, qRE4 and qRE5 were still detected by using the LMM model (Figure 1b). While there were 37 QTL detected as associated with root elongation without Zn toxicity, the same QTL were not found with root elongation under high Zn toxicity and without Zn toxicity (Table 2 and Table S2).
Table 2.
Novel QTL for root elongation under high Zn toxicity in Ting’s core collection.
| QTL | Chromosome | Position | p-Value | Allele (the Reference/the Alternative) |
|---|---|---|---|---|
| qRE1 | chr02 | 20,681,680 | 5.34 × 10−7 | A/G |
| qRE2 | chr02 | 26,288,978 | 3.98 × 10−6 | A/G |
| qRE3 | chr06 | 1,118,739 | 2.35 × 10−6 | A/T |
| qRE4 | chr06 | 25,006,144 | 9.09 × 10−6 | T/C |
| qRE5 | chr08 | 23,227,944 | 5.70 × 10−7 | T/C |
| qRE6 | chr09 | 7,341,101 | 8.07 × 10−8 | T/C |
| qRE7 | chr11 | 27,019,946 | 4.12 × 10−6 | G/T |
RE: root elongation.
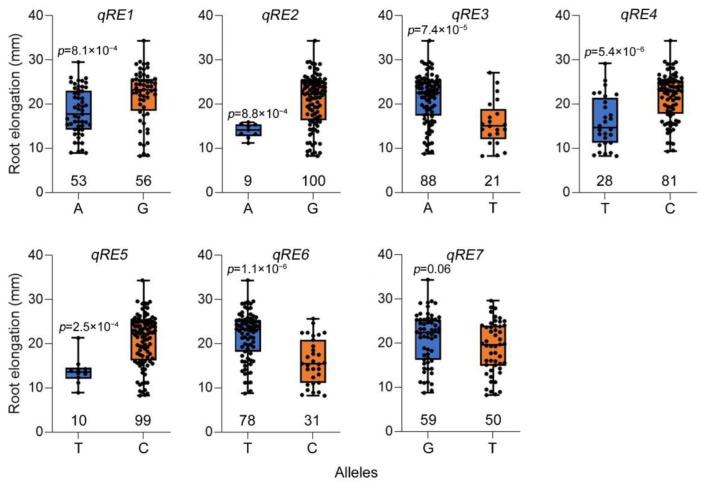
Additionally, we focused on QTL associated with root elongation under high Zn conditions. The most significant SNP for each QTL was selected to detect the effects of allelic variations on root elongation under high Zn toxicity (Figure 2 and Table 2). Under high Zn toxicity, the root elongation of varieties with reference (Nipponbare) alleles was significantly larger than those with alternative alleles in Ting’s core collection, whereby the most significant SNPs of qRE1, qRE2, qRE4, and qRE5. However, the root elongation of varieties with reference (Nipponbare) alleles was significantly smaller than those with alternative alleles in Ting’s core collection, whereby the most important SNPs of qRE3 and qRE6 (Figure 2).
Figure 2.
Effect analysis of allelic variations on root elongation under high Zn toxicity.
2.3. GWAS for Relative Root Elongation under High Zn Toxicity within Ting’s Core Collection
We then focused on the QTL associated with relative root elongation. In the present study, 19 novel QTL distributed on all chromosomes, except chr05, were identified as significantly (p < 0.94 × 10−5) associated with root elongation under high Zn toxicity by using the EMMAX model (Table 3). Among them, qRRE4, qRRE8 and qRRE9 were detected by using the LMM model (Figure 1d). Furthermore, there were two QTL, i.e., qRE4 and qRRE9, located in the same region on chromosome 6 (Figure 1b, d). Moreover, qRRE1 was located in the region of OsMTI-3a, which was reported to relate to Zn tolerance in yeast (Table 3), and qRRE15 was identical to three previous QTL, i.e., qZRSDW11-2, qZRTDW11, and qZnRL11 (Figure 1d).
Table 3.
QTL for relative root elongation identified in Ting’s core collection.
| QTL | Chromosome | Position | p-Value | Allele (the Reference/the Alternative) | Previously Reported QTL |
|---|---|---|---|---|---|
| qRRE1 | chr01 | 5,483,287 | 3.43 × 10−6 | A/G | OsMTI-3a [28] |
| qRRE2 | chr01 | 18,578,810 | 1.84 × 10−6 | A/G | |
| qRRE3 | chr02 | 7,969,392 | 3.50 × 10−6 | G/A | |
| qRRE4 | chr03 | 13,749,414 | 3.16 × 10−7 | G/A | |
| qRRE5 | chr03 | 19,713,560 | 8.19 × 10−7 | C/T | |
| qRRE6 | chr03 | 25,346,195 | 1.22 × 10−6 | T/C | |
| qRRE7 | chr04 | 17,157,142 | 1.56 × 10−6 | G/A | |
| qRRE8 | chr06 | 17,185,339 | 4.76 × 10−7 | A/G | |
| qRRE9 | chr06 | 25,053,430 | 3.29 × 10−7 | G/A | |
| qRRE10 | chr07 | 8,451,115 | 2.59 × 10−6 | C/T | |
| qRRE11 | chr08 | 22,766,099 | 2.45 × 10−6 | T/C | |
| qRRE12 | chr08 | 28,240,531 | 2.06 × 10−6 | T/G | |
| qRRE13 | chr09 | 5,323,335 | 2.45 × 10−6 | C/T | |
| qRRE14 | chr10 | 22,657,213 | 3.96 × 10−6 | A/T | |
| qRRE15 | chr11 | 8,988,532 | 3.02 × 10−6 | C/G |
qZRSDW11-2 qZRTDW11 [14]; qZnRL11 [15] |
| qRRE16 | chr11 | 10,391,796 | 5.15 × 10−7 | C/T | |
| qRRE17 | chr11 | 27,270,465 | 1.45 × 10−6 | C/T | |
| qRRE18 | chr12 | 22,393,995 | 3.33 × 10−7 | G/A | |
| qRRE19 | chr12 | 23,423,605 | 3.60 × 10−6 | A/G |
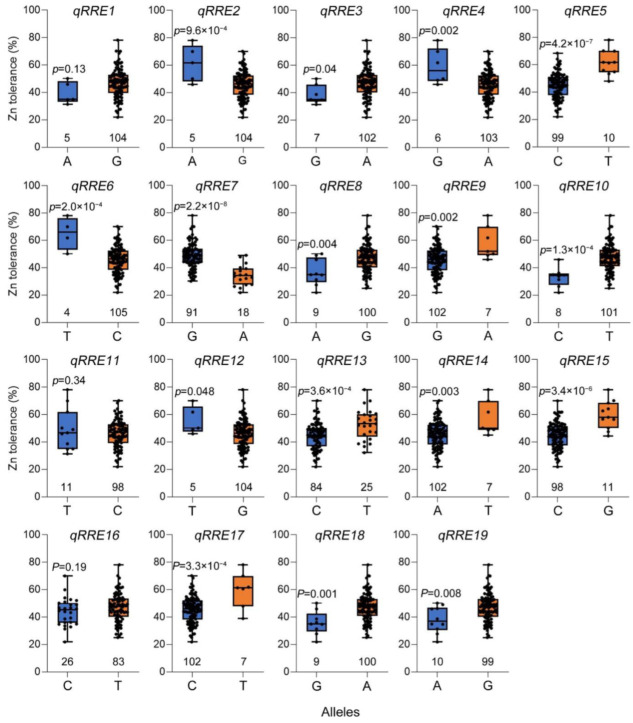
The most significant SNP for each QTL was selected for detecting the effects of allelic variations on relative root elongation under high Zn toxicity (Figure 3 and Table 3). Under high Zn toxicity, the varieties with reference (Nipponbare) alleles were significantly more tolerant than those with alternative alleles, which were grouped by the most significant SNPs of qREE2, qRRE4, qRRE6, qRRE7, and qRRE12. However, the varieties with reference (Nipponbare) alleles were significantly more sensitive than those with alternative alleles in Ting’s core collection, which were grouped by the most significant SNPs of qRRE3, qRRE5, qRRE8, qRRE9, qRRE10, qRRE13, qRRE14, qRRE15, qRRE17, qRRE18, and qRRE19 (Figure 3).
Figure 3.
Effect analysis of allelic variations on relative root elongation under high Zn toxicity.
2.4. Candidate Genes Analysis of Detected QTL Responding to Zn Toxicity in the Present Study
After detecting QTL associated with Zn toxicity tolerance, we sought to isolate the genes that regulate Zn tolerance in rice. We analyzed the candidate genes of QTL (Table S3) according to the following reasons: firstly, we chose QTL detected using both the EMMAX and LMM models, and qRE5, qRE4/qRRE9, qRRE4 as well as qRRE8 were detected both in EMMAX and LMM; secondly, we selected QTL that were identical to previous QTL, i.e., qRRE15; and thirdly, the genes in Fe homeostasis, Zn chelator, phosphate transporters, and MATE efflux families have been shown to be related to Zn tolerance, and we found that there were many genes in the above families annotated in the regions of qRE2, qRRE1, and qRRE12.
On the basis of the above reasons, the information about candidate genes is detailed as follows: For qRE2 on chromosome 2, there were 25 candidate genes in the region 26.20–26.39 Mb. Among these candidate genes, one gene (Os02g0650300) was an iron (III)–deoxymugineic acid transporter (OsYSL15), which is responsible for iron uptake and the phloem transport of iron [26,27]. For qRRE1 on chromosome 1, there were 29 candidate genes in the region 5.38–5.59 Mb. One candidate was Os01g0200700 (OsMTI-3a), which is involved in the detoxification of Zn, Ni, and Cd [28]. For qRRE4 on chromosome 3, there was only one significant SNP and 17 candidate genes in the region 13.65–13.85 Mb (Figure 4a). For qRRE8 on chromosome 6, there were 15 candidate genes in the region 17.13–17.29 Mb, which were covered by four significant SNPs (Figure 4b). One candidate was a phosphate transporter (OsPHO1;3), and two candidates were from the MATE efflux family. The most significant peak (qRRE9) of all these loci was located on chromosome 6 around the top SNP at position 25,053,430. The candidate region was from 24.92 Mb to 25.12 Mb (200 kb) by LD calculation and contained twenty-two candidate genes, which were covered by 35 significantly associated SNPs (Figure 4c). For qRRE12, there were 26 candidate genes in the region 28.14–28.34 Mb. One candidate gene was Os08g0564000 (OsPT6), which plays a role in the uptake and the long-distance transport of Pi from roots to shoots, [29] and another one was a MATE efflux family protein. For qRRE15, there were sixteen candidate genes in the region 8.88–9.08 Mb, which were covered by eight significant SNPs.
Figure 4.
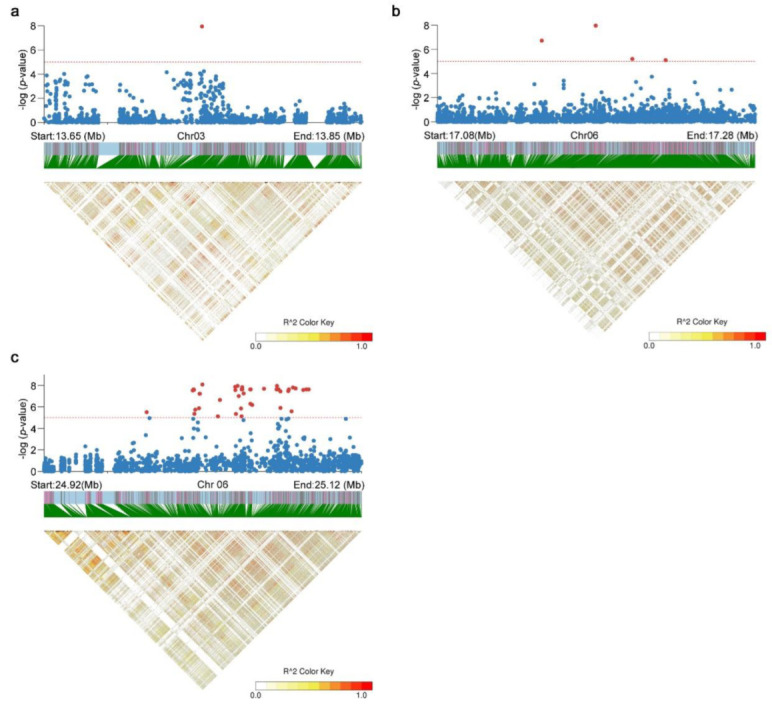
Local Manhattan plot (top panel) and LD heatmap (bottom panel) of the 13.65–13.85 Mb region of chromosome 3 (a), 17.08–17.28 Mb region of chromosome 6 (b), and 24.91–25.11 Mb region of chromosome 6 (c).
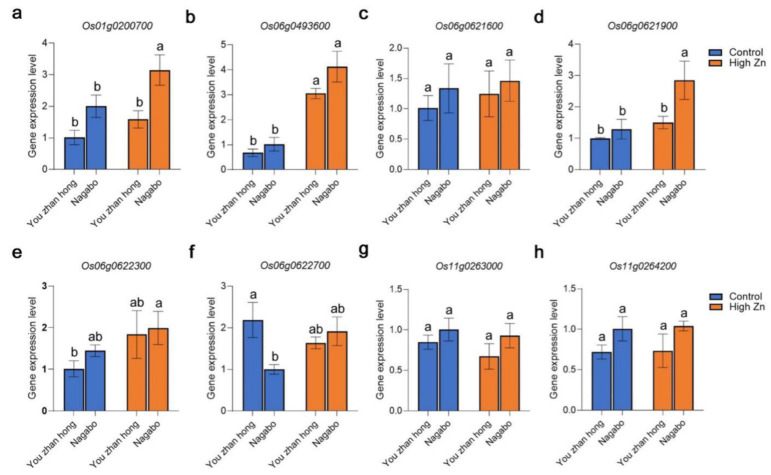
Furthermore, eight candidate genes detected by GWAS were selected to verify by qRT-PCR. Among the eight candidate genes, the expression of three genes was significantly different in the roots between Nagabo (Zn tolerant) and You zhan hong (Zn sensitive) (Figure 5a,d,f) under Zn toxicity. While the expression of the gene in Figure 5b was induced by Zn toxicity, the expression of the remaining four candidate genes was not significant between Zn-tolerant and Zn-sensitive varieties, nor was it induced by Zn toxicity (Figure 5c,e,g,h).
Figure 5.
The gene expression of 8 candidate genes in representative varieties (the most sensitive variety in the present study, You zhan hong; the most tolerant variety in the present study, Nagabo) in control and high Zn conditions as measured by qRT-PCR. Error bars: S.D. The different letters indicate significant differences by one-way ANOVA analysis with Tukey’s HSD test (p < 0.05, n = 3).
3. Discussion
Ting’s core collection may be a potential resource of elite genes/alleles for Zn tolerance in rice due to abundant and useful natural variations for agronomic traits, aluminum tolerance, as well as rice sheath blight resistance, which have been reported within this core collection in our previous studies [22,23,24,25]. In fact, the extension of phenotypic variations for Zn tolerance within Ting’s core collection is large in the present study (Figure 1a,c, Table 1). Moreover, rice varieties with strong Zn tolerance were discovered in the present study (Table S1).
The concentrations of Zn toxicity used in previous studies were diverse due to different populations and methods, e.g., 300 mg/L and 200 mg/L were used in the studies of Meng et al. (2017) [15] and Zhang et al. (2017) [16]. Moreover, we do not think that positive QTL regarding Zn tolerance will be identified by using an unsuitable Zn toxicity concentration. Thus, several concentrations (50 μM, 120 μM, 150 μM, 200 μM, and 300 μM, data not shown) of Zn toxicity were used to determine suitable treatment concentration, and a concentration of 120 μM was distinct for Zn tolerance among Ting’s core collection. Furthermore, the concentration was proven suitable, from the results of this GWAS, because identical QTL were not found in root elongation under high Zn toxicity and without Zn toxicity (Table 2 and Table S2). The above evidence supports the results of this GWAS.
Furthermore, a number of novel QTL, which are identical to previous QTL, and previously identified QTL were uncovered in this GWAS. A total of 4,395,003 high-quality SNPs were used to perform this GWAS using the EMMAX and LMM models. The above super-density SNPs covered most of the genomic regions for Ting’s core collection; thus, the results of the GWAS in the present study may offer reliability and accuracy in LD decay distance, population structure, and kinship value, which should have been the requirements of the GWAS in our previous studies [22,23,24,25]. Indeed, we can only speculate facts based on the above-mentioned information. In the present study, we found that all significant QTL identified by EMMAX were also detected by LMM, and three QTL (qRRE4, qRRE8, and qRRE9) using relative root elongation under Zn toxicity exceeded the supposedly stringent significance threshold (−log10(1/n) = 7.64, Figure S2b) when the LMM model was used. For qRRE4 and qRRE8, there were only one and four SNPs, respectively, within a candidate region of 200 kb distance surrounding the most significant SNP. For qRRE9, there were thirty-five SNPs surrounding the most significant SNP at position 25,053,430 on chromosome 6 (Figure 4c). We speculate that it is worthwhile to dedicate more works on qRRE9 in the future because obvious LD decay was uncovered in this QTL (Figure 4c).
Furthermore, in the present study, the same QTL were detected by using different phenotypes, as well as identified in previous studies. When comparing QTL detected by root elongation and relative root elongation under high Zn toxicity, we found that qRE4 was located in the same region as qRRE9 (Figure 1). When comparing QTL identified in the present study (Table 2 and Table 3) with previously reported Zn-tolerant QTL, we found that qRRE15, which is located on chromosome 11, was mapped in the same region as QTL (qZRSDW11-2 and qZRTDW11) detected by relative shoot dry weight and relative total dry weight using reciprocal introgression populations [14] and a QTL (qZnRL11) detected by root length using MAGIC populations [15]. These results suggest that qRE4/qRRE9 and qRRE15 may be the causal loci involved in Zn toxicity tolerance.
Although many QTL associated with Zn toxicity tolerance in rice have been detected using different traits [12,14,15,16], the major quantitative genetic variants have not yet been fine-mapped or cloned, which means little is known about the genetic mechanisms of rice tolerant to Zn toxicity. Using high-density SNPs and natural variations in root elongation for GWAS analysis, we detected a number of novel QTL.
There is already vast knowledge on the molecular mechanisms of Zn homeostasis in plants, which was summarized in a review of Kaur and Garg (2021) [30]. Previous studies have shown that excess Zn caused Fe deficiency-induced chlorosis through chlorophyll synthesis reduction, chloroplast degradation, and reduced P levels in shoots [2]. Moreover, AtFRD3, which encodes a member of the MATE efflux family, has been demonstrated to play a role in cross homeostasis between Fe and Zn tolerance in Arabidopsis [11]. Yellow stripe 1-like (YSL) genes encode transporters, which can transport iron, Zn, and nickel NA complexes [31]. Transgenic plants and yeasts that carry OsMT1a accumulate more Zn than wild type [32]. Thus, we further analyzed the candidate genes in the region of some loci, which included the genes in Fe homeostasis, Zn chelator, phosphate transporters, and the MATE efflux family (Table S3). In the present study, we detected OsYSL15 located in the region of qRE2, OsMTI-3a in the region of qRRE1, OsPHO1;3 and two MATE efflux-related genes in the region of qRRE8, and OsPT6, as well as a MATE efflux gene, in the region of qRRE12. In addition, qRT-PCR was performed to support the candidate genes identified in the present study; we will deeply study four candidate genes, i.e., Os01g0200700, Os06g0621900, Os06g0493600, and Os06g0622700, by using technology such as CRISPR-Cas9 and over-expression. Therefore, the above results suggest that these genes might be the causal genes that respond to Zn toxicity. However, the molecular mechanism of tolerance under Zn toxicity is complicated; hence, further studies are necessary to confirm the candidate genes and reveal the molecular mechanisms of rice adaptation to Zn toxicity.
Moreover, Zn-tolerant (Nagabo, Duan mang zi jin gu, and Bu gou wei) and Zn-sensitive varieties (You zhan hong) are suitable genetic resources for improving the Zn tolerance of rice cultivars. Therefore, the tolerant elite alleles in these varieties can be pyramided by marker-assisted breeding or edited directly by CRISPR-Cas9 in an elite, modern cultivar background.
4. Materials and Methods
4.1. Plant Materials
Ting’s core collection, which consisted of 150 rice landraces, was collected from China, Korea, Japan, the Philippines, Java, Oceania, Vietnam, and Brazil [21] (Table S1).
4.2. Phenotyping for Zn Toxicity Tolerance
The seeds of Ting’s core collection were harvested from the field of China National Rice Research Institute, Hangzhou in 2020. The seeds were soaked in water for 2 days at 30 °C in the dark, transferred to a 1 L pot with 96-well PCR plates (8 × 12) in a solution containing 0.5 mM CaCl2 (pH = 5.6). After 1 day, the seedlings were subjected to a solution of 0.5 mM CaCl2 containing 0 (control) and 120 μM ZnSO4 for 24 h. The experiment was conducted using a randomized complete block design with three replicates and 6 uniformly germinated seeds. The root length (RE) was measured by a ruler before and after the Zn treatment. Relative root elongation (RRE) was calculated based on (root elongation in high Zn/ root elongation in control conditions) × 100 [33]. The screening for Zn was conducted in a growth chamber with day/night temperature of 30/28 °C and relative humidity of 75%.
4.3. SNPs Calling
The 4,395,003 SNPs used for GWAS were based on re-sequencing performed by Illumina HiSeqTM 4000 with 6~7-fold of genome coverage; detailed information was described in our previous study [22,24]. The SNPs with low minor allele frequency (0.05) were removed, and, finally, 4,393,003 SNPs were selected for our GWAS analysis.
4.4. GWAS Analysis
We performed GWAS to detect the trait–SNP associations for Zn tolerance using 4,395,003 SNPs and the mean value and tolerance index of root elongation at high Zn. Two mixed linear models were used, the EMMAX and LMM methods. The critical p-value threshold for identifying the significant marker–trait associations in our study was 2.27 × 10−7 (p = 1/n; n = total markers), which is a rough Bonferroni correction corresponding to −log10(p) = 7.64. However, when there was no peak detected, another significance threshold was calculated, i.e., a minimum Bayes factor (mBF). The mBF was calculated using the following formula: mBF = −e*p*ln(p) [22], thus, the significance threshold in present study was -log10(mBF) = 5.02. Moreover, the peaks exhibiting a significance threshold level within a physical distance of about 200 kb were merged into a single QTL.
4.5. qRT-PCR
The seedlings were grown in 0.5 mM CaCl2 solution (pH = 5.6) for 1 day after germination, and then subjected to a 0.5 mM CaCl2 solution containing 0 μM (control) and 120 μM (high Zn) Zn concentrations for 12 h. Whole roots were harvested and immediately frozen in liquid nitrogen. Total RNA was extracted using the FastPure Universal Plant Total RNA Isolation Kit (Vazyme, Nanjing, China). First-strand cDNA was synthesized using the ABScrip III RT Master Mix (ABclonal, Wuhang, China). qRT-PCR reaction was prepared using the THUNDERBIRD SYBR qPCR Mix (TOYOBO, Tokyo, Japan). Three biological replicates and two technical replicates were analyzed for each gene. Relative expression of all genes was normalized using OsUBQ1 (Os03g0234350) as an internal reference. The primers used for qRT-PCR are shown in Table S4.
5. Conclusions
In the present study, a total of 7 and 19 QTL were identified as significantly associated with Zn tolerance using root elongation (RE) and relative root elongation (RRE) under high Zn toxicity. Among them, qRE4, which was detected at high Zn concentrations, was located in the same region as qRRE9. qRRE15 was located in the same region of three previously reported QTL (qZnRL11, qZRSDW11-2, and qZRTDW11). These results indicate that there may be causal genes regulating Zn tolerance in the above-mentioned QTL. Furthermore, a number of candidate genes were detected in QTL in the present study, including the genes involved in Fe homeostasis, metal chelators, and Pi transporters. Moreover, eight candidate genes were verified through qRT-PCR. Three varieties (Nagabo, Duan mang zi jin gu, and Bu gou wei) had elite alleles identified in the QTL associated with high Zn tolerance. Taken together, our findings provide significant insight into the genetic basis for Zn toxicity tolerance and will be very useful for developing rice varieties that are tolerant to Zn toxicity and improving rice production in Zn-contaminated soils.
Acknowledgments
Thanks to the anonymous reviewers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11223138/s1, Figure S1: GWAS of root elongation in control conditions, a distribution of root elongation of Ting’s core collection grown in control conditions, b: box plots for root elongation under high Zn (120 μM) conditions and without Zn toxicity. The P-value of Student’s t-test was shown for comparing root elongation under high and without Zn toxicity, c: Manhattan plot of GWAS using root elongation without Zn toxicity. Chromosomes are depicted in different colors. The green line depicts the adjusted significance threshold (p = 0.94 × 10−5), Figure S2: GWAS of Zn tolerance in high Zn conditions using LMM model, a: Manhattan plot of GWAS using root elongation in high Zn conditions, b: Manhattan plot of GWAS using relative root elongation in high Zn conditions. Chromosomes are depicted in different colors. The horizontal red line depicts the significance threshold (p = 2.27 × 10−7), and the green line depicts the adjusted significance threshold (p = 0.94 × 10−5). The black arrows represent the significant loci detected using EMMAX and LMM models. The red arrow represents the same loci as the previously reported under Zn stress, Table S1: root growth of Ting’s core collection in control and Zn toxicity conditions, Table S2: QTL for root elongation of Ting’s core collection in control condition, Table S3: candidate genes associated with Zn tolerance, Table S4: primers used for qRT-PCR.
Author Contributions
Conceptualization, K.Z., P.Z., X.W. and P.H.; methodology, K.Z. and P.Z.; software, K.Z. and P.Z.; validation, K.Z., P.Z. and P.H.; formal analysis, K.Z. and P.Z.; investigation, K.Z., L.X., S.H., G.S., Z.S., G.J., L.W., Y.C. and S.T.; resources, P.Z.; data curation, K.Z., S.H., S.T. and G.S.; writing—original draft preparation, K.Z. and P.Z.; writing—review and editing, K.Z., P.Z., X.W. and P.H.; visualization, K.Z.; supervision, P.H.; project administration, P.Z., X.W. and P.H.; funding acquisition, P.Z. and P.H. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the fund of Hainan Provincial Joint Project of Sanya Yazhou Bay Science and Technology City, grant number 2021JJLH0041 and the fund of Hainan Province Science and Technology Special Fund, grant number ZDYF2022XDNY256.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Broadley M.R., White P.J., Hammond J.P., Zelko I., Lux A. Zinc in plants. New Phytol. 2012;173:677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- 2.Chaney R.L. Zinc phytotoxicity. In: Robson A.D., editor. Zinc in Soil and Plants. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1993. pp. 135–150. [Google Scholar]
- 3.Hussain D., Haydon M.J., Wang Y.W., Wong E., Sherson S.M., Young J., Camakaris J., Harper J.F., Cobbett C.S. P-Type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell. 2004;16:1327–1339. doi: 10.1105/tpc.020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verret F., Gravot A., Auroy P., Leonhardt N., David P., Nussaume L., Vavasseur A., Richaud P. Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett. 2004;576:306–312. doi: 10.1016/j.febslet.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Hanikenne M., Talke I.N., Haydon M.J., Lanz C., Nolte A., Motte P., Kroymann J., Weigel D., Krämer U. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature. 2008;453:391–395. doi: 10.1038/nature06877. [DOI] [PubMed] [Google Scholar]
- 6.Song W.Y., Choi K.S., Kim D.Y., Geisler M., Park J.Y., Vincenzetti V., Schellenberg M., Kim S.H., Lim Y.P., Noh E.W., et al. Arabidopsis PCR2 is a zinc exporter involved in both zinc extrusion and long-distance zinc transport. Plant Cell. 2010;22:2237–2252. doi: 10.1105/tpc.109.070185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobae Y., Uemura T., Sato M.S., Ohnishi M., Mimura T., Nakagawa T., Maeshima M. Zinc transporter of Arabidopsis thaliana AtMTP1 is localized to vacuolar membranes and implicated in zinc homeostasis. Plant Cell Physiol. 2004;45:1749–1758. doi: 10.1093/pcp/pci015. [DOI] [PubMed] [Google Scholar]
- 8.Desbrosses-Fonrouge A.G., Voigt K., Schröder A., Arrivault S., Thomine S., Krämer U. Arabidopsis thaliana MTP1 is a Zn transporter in the vacuolar membrane which mediates Zn detoxification and drives leaf Zn accumulation. FEBS Lett. 2005;579:4165–4174. doi: 10.1016/j.febslet.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 9.Arrivault S., Senger T., Krämer U. The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. Plant J. 2006;46:861–879. doi: 10.1111/j.1365-313X.2006.02746.x. [DOI] [PubMed] [Google Scholar]
- 10.Haydon M.J., Cobbett C.S. A novel major facilitator superfamily protein at the tonoplast influence zinc tolerance and accumulation in Arabidopsis. Plant Physiol. 2007;143:1705–1719. doi: 10.1104/pp.106.092015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pineau C., Loubet S., Lefoulon C., Chalies C., Fizames C., Lacombe B., Ferrand M., Loudet O., Berthomieu P., Richard O. Natural variation at the FRD3 MATE transporter locus reveals cross-talk between Fe homeostasis and Zn Tolerance in Arabidopsis thaliana. PLoS Genet. 2012;8:e1003120. doi: 10.1371/journal.pgen.1003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Y.J., Ogawa T., Lin D.Z., Koh H.J., Kamiunten H., Matsuo M., Cheng S.H. Molecular mapping of quantitative trait loci for zinc toxicity tolerance in rice seedling (Oryza sativa L.) Field Crops Res. 2006;95:420–425. doi: 10.1016/j.fcr.2005.03.005. [DOI] [Google Scholar]
- 13.Song A.L., Li P., Li Z.J., Fan F.L., Nikolic M., Liang Y.C. The alleviation of zinc toxicity by silicon is related to zinc transport and antioxidative reactions in rice. Plant Soil. 2011;344:319–333. doi: 10.1007/s11104-011-0749-3. [DOI] [Google Scholar]
- 14.Liu H., Soomro A., Zhu Y.J., Qiu X.J., Chen K., Zheng T.Q., Yang L.W., Xing D.Y., Xu J.L. QTL underlying iron and zinc toxicity tolerances at seedling stage revealed by two sets of reciprocal introgression populations of rice (Oryza sativa L.) Crop. J. 2016;4:280–289. doi: 10.1016/j.cj.2016.05.007. [DOI] [Google Scholar]
- 15.Meng L.J., Wang B.X., Zhao X.Q., Ponce K., Qian Q., Ye G.Y. Association mapping of ferrous, zinc, and aluminum tolerance at the seedling stage in indica rice using MAGIC populations. Front. Plant Sci. 2017;8:1822. doi: 10.3389/fpls.2017.01822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J., Chen K., Pang Y.L., Naveed S.A., Zhao X.Q., Wang X.Q., Wang Y., Dingkuhn M., Pasuquin J., Li Z.K., et al. QTL mapping and candidate gene analysis of ferrous iron and zinc toxicity tolerance at seedling stage in rice by genome-wide association study. BMC Genom. 2017;18:828. doi: 10.1186/s12864-017-4221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkins D.A. The measurement of tolerance to edaphic factors by means of root growth. New Phytol. 1978;80:623–633. doi: 10.1111/j.1469-8137.1978.tb01595.x. [DOI] [Google Scholar]
- 18.Platre M.P., Satbhai S.B., Brent L., Gleason M.F., Cao M., Grison M., Glavier M., Zhang L., Gaillochet C., Goeschl C., et al. The receptor kinase SRF3 coordinates iron-level and flagellin dependent defense and growth responses in plants. Nat. Commun. 2022;13:4445. doi: 10.1038/s41467-022-32167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B.H., Sun L., Huang J.Y., Göschl C., Shi W.M., Chory J., Busch W. GSNOR provides plant tolerance to iron toxicity via preventing iron-dependent nitrosative and oxidative cytotoxicity. Nat. Commun. 2019;10:3896. doi: 10.1038/s41467-019-11892-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satbhai S., Setzer C., Freynschlag F., Slovak R., Kerdaffrec E., Busch W. Natural allelic variation of FRO2 modulates Arabidopsis root growth under iron deficiency. Nat. Commun. 2017;8:15603. doi: 10.1038/ncomms15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X.L., Lu Y.G., Li J.Q., Muhammad Q. Strategies on sample size determination and qualitative and quantitative traits integration to construct core collection of rice (Oryza sativa) Rice Sci. 2011;18:46–55. doi: 10.1016/S1672-6308(11)60007-3. [DOI] [Google Scholar]
- 22.Zhang P., Zhong K.Z., Zhong Z.Z., Tong H.H. Mining candidate gene for rice aluminum tolerance through genome wide association study and transcriptomic analysis. BMC Plant Biol. 2019;19:490. doi: 10.1186/s12870-019-2036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P., Zhong K.Z., Tong H.H., Qasim M., Li J.Q. Association mapping for aluminum tolerance in a core collection of rice landraces. Front. Plant Sci. 2016;7:1415. doi: 10.3389/fpls.2016.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang P., Zhong K.Z., Zhong Z.Z., Tong H.H. Genome-wide associated study of important agronomic traits within a core collection of rice (Oryza sativa L.) BMC Plant Biol. 2019;19:259. doi: 10.1186/s12870-019-1842-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu D., Zhong K.Z., Zhong Z.Z., Hu G.C., Zhang P., Tong H.H. Genome-wide association study of sheath blight resistance within a core collection of rice (Oryza sativa L.) Agronomy. 2022;12:1493. doi: 10.3390/agronomy12071493. [DOI] [Google Scholar]
- 26.Lee S., Chiecko J.C., Kim S.A., Walker E.L., Lee Y.S., Guerinot M.L., An G. Disruption of OsYSL15 leads to iron inefficiency in rice plants. Plant Physiol. 2009;150:786–800. doi: 10.1104/pp.109.135418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue H., Kobayashi T., Nozoye T., Takahashi M., Kakei Y., Suzuki K., Nakazono M., Nakanishi H., Mori S., Nishizawa N.K. Rice OsYSL15 is an iron-regulated iron (III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J. Biol. Chem. 2009;284:3470–3479. doi: 10.1074/jbc.M806042200. [DOI] [PubMed] [Google Scholar]
- 28.Shahpiri A., Soleimanifard I., Asadollahi M.A. Functional characterization of a type 3 methallolthionein isoform (OsMTI-3a) from rice. Int. J. Biol. Macromol. 2015;73:154–159. doi: 10.1016/j.ijbiomac.2014.10.067. [DOI] [PubMed] [Google Scholar]
- 29.Ai P.H., Sun S.B., Zhao J.N., Fan X.R., Xin W.J., Guo Q., Yu L., Shen Q.R., Wu P., Miller A.J., et al. Two rice phosphate transporters, OsPht1;2 and OsPht1;6 have different functions and kinetic properties in uptake and translocation. Plant J. 2009;57:798–809. doi: 10.1111/j.1365-313X.2008.03726.x. [DOI] [PubMed] [Google Scholar]
- 30.Kaur H., Garg N. Zinc toxicity in plants: A review. Planta. 2021;253:129. doi: 10.1007/s00425-021-03642-z. [DOI] [PubMed] [Google Scholar]
- 31.Curie C., Cassin G., Couch D., Divol F., Higuchi K., Jean M.L., Misson J., Schikora A., Czernic P., Mari S. Metal movement within the plant: Contribution of nicotianamine and yellow stripe 1-like transporter. Ann. Bot. 2009;103:1–11. doi: 10.1093/aob/mcn207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Z., Wu Y.R., Li Y., Ling H.Q., Chu C.C. OsMT1a, a type 1 metallothionein, plays the pivotal role in zinc homeostasis and drought tolerance in rice. Plant Mol. Biol. 2009;70:219–229. doi: 10.1007/s11103-009-9466-1. [DOI] [PubMed] [Google Scholar]
- 33.Huang S., Ma J.F. Silicon suppresses zinc uptake through down-regulating zinc transporter gene in rice. Physiol. Plantarum. 2020;170:580–591. doi: 10.1111/ppl.13196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.