Abstract
Background
Central sensitization syndrome (CSS) involves severe functional symptoms due to central sensitization. for patients with severe somatic symptoms and related disorders (SSRDs), central sensitization may be responsible for their functional symptoms. We hypothesized that screening for CSS in patients with SSRDs would identify those with severe disease. The Somatic Symptom Scale-8 (SSS-8) is a simple tool to assess medical conditions related to SSRDs, but the cut-off point to identify severe cases of comorbid CSS is unknown. This study aimed to determine the optimal cut-off point of SSS-8 for screening the CSS of patients with severe SSRDs.
Methods
In total, 143 patients with SSRDs attending outpatient clinics of a university hospital in Japan were included in the study. The participants were evaluated using the SSS-8 for somatic symptoms, Hospital Anxiety and Depression Scale (HADS) for anxiety and depressive symptoms, Pain Catastrophizing Scale (PCS) for catastrophic thoughts, and Central Sensitization Inventory (CSI-A, B) for CSS. Receiver operating characteristic (ROC) curve analysis was performed using the propensity score. The area under the curve (AUC) was calculated using a propensity score considering PCS, age, sex, HADS, and CSI-B as confounders of SSS-8 and CSS to evaluate differences in diagnostic accuracy between patients with and without SSS-8. The sensitivity and specificity of the ROC analysis were then used to determine the cut-off point for discriminating severe cases of SSS-8.
Results
Of the 143 participants, 126 responded (51 CSS group and 75 non-CSS group), with a valid response rate of 88.1 percent. In the ROC analysis, the propensity score including SSS-8 was statistically more accurate. The optimal cut-off point was 13, with an AUC of 0.88, sensitivity of 84.3 percent, and specificity of 77.3 percent.
Conclusions
The SSS-8 is a useful tool for discriminating severe cases of SSRDs comorbid with CSS.
Keywords: Central sensitization syndrome, Somatic symptom disorder, Somatic Symptom Scale-8
Background
The somatic symptoms observed in patients with somatic symptom and related disorders (SSRDs) [1] are not based on fatal organic abnormalities [2] and are considered to reflect a complex syndrome of biological, psychological, and social problems [3]. Patients with SSRDs commonly present with multiple somatic symptoms, and the Patient Health Questionnaire-15 (PHQ-15) is used to assess these symptoms [4]. Additionally, the Somatic Symptom Scale-8 (SSS-8) was developed to more easily assess the disease status of SSRDs [5]. The SSS-8 was developed as a short form of the PHQ-15, and is used in clinical practice for follow-up of medical conditions in primary care [6] and psychosomatic outpatient clinics [7].
In patients with SSRDs, pharmacotherapy and psychotherapy have been attempted to treat the somatic symptoms and psychological problems of the patients [8]. However, in such patients, functional somatic symptoms typically persist for more than 6 months [1] and are particularly difficult to treat in severe cases [9, 10]. A previous study has reported that functional somatic symptoms are most severe when affected by central sensitization in particular [11, 12]. Central sensitization is defined as a neurophysiological condition in which hyperexcitability of the central nervous system induces hyperalgesia [13] and affects psychological factors such as catastrophic thoughts [14]. Conditions that are strongly influenced by central sensitization are comprehensively treated as central sensitization syndrome (CSS) [13].
Hence, screening for CSS in patients with SSRDs would be useful for identifying those with severe SSRDs. The severity of SSRDs are classified into five levels when using the SSS-8 scores [5]. However, in busy clinical practice, the SSS-8 would serve as a more convenient screening tool by setting a cut-off point for the SSS-8 to discriminate severe cases. The aim of this study was to determine the optimal cut-off point of SSS-8 for the screening of CSS among patients with severe SSRDs.
Methods
Participants
The study was cross-sectional. Participants were recruited from among the patients who visited the Department of Psychosomatic Medicine at Toho University Medical Center Omori Hospital between February and March 2021. The inclusion criteria were as follows: 1) age 20–79 years; 2) accurate understanding of the purpose and process of the study and signing an informed consent form; 3) meeting the diagnostic criteria for SSRDs [1]. Exclusion criteria included diagnosis of 1) schizophrenia spectrum disorder and other psychotic disorders; 2) dementia (such as Alzheimer's dementia, vascular dementia, Parkinson's disease dementia, and Lewy body dementia); 3) neurodevelopmental disorders (such as autism spectrum disorder, attention deficit/hyperactivity disorder, communication disabilities); 4) dissociative disorders; and 5) patients who for any reason could not be accurately assessed.
The diagnoses were made by multiple physicians using the Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-5) [1]. Data regarding age, sex, education, and duration of treatment were collected as background factors from all participants.
Questionnaires
The SSS-8 [5] was used to assess somatic symptoms; the Japanese version of the SSS-8 [15] has been validated linguistically and psychologically and has internal consistency [16].
The Central Sensitization Inventory (CSI) [17] was used to assess central sensitization. The CSI consists of two parts. Part A assesses subjective symptoms common to CSS and Part B asks whether the subject has had CSS in the past. CSI is a questionnaire with high reliability and internal consistency, and the reliability and validity of the Japanese version of CSI have already been verified in a previous study [18]. The CSI correlates with quantitative sensory tests used for inferring CSS [19, 20], and a cut-off point of 40 or higher on the CSI-A has been reported to be useful for discriminating CSS in outpatient clinics [21]. In this study, patients with a CSI-A of 40 points or higher were included in the CSS group.
We assessed the participants' state of anxiety, depression, and catastrophic thinking, which are psychological states that have been reported to be related to central sensitization in previous studies [22–24].
The Hospital Anxiety and Depression Scale (HADS) [25] is a questionnaire consisting of seven items each on anxiety and depression. Both the anxiety and depression scales are scored from 0 to 21 points and are used as clinical indicators of psychiatric symptoms in general practice [26]. The HADS has also been reported to be associated with quality of life [27], and the Japanese version of the HADS has been validated for reliability and validity [28]. The Pain Catastrophizing Scale (PCS) [29] is a 13-item questionnaire with three subscales (rumination, helplessness, and magnification) that assesses catastrophic thinking and has shown high reliability and validity. The reliability and validity of the Japanese version of the PCS were also confirmed [30].
Data analysis
For differences in background factors and endpoints between the CSS and non-CSS groups, nominal variables were subjected to chi-square or Fisher’s test, continuous variables to t-test, and categorical variables and non-normally distributed continuous variables to Mann–Whitney U test.
To evaluate the utility of the SSS-8 in discriminating between the CSS and non-CSS groups, two propensity scores were calculated by logistic regression analysis. One was the propensity score with CSS as the dependent variable, SSS-8 as the independent variable, and PCS, HADS, age, sex, and CSI-B as confounders of CSS, and the other was the propensity score with CSS as the dependent variable and PCS, HADS, age, sex, and CSI-B as independent variables. Receiver operating characteristic (ROC) curve analyses were performed on the propensity scores [31] to statistically compare the area under the curve (AUC) with and without SSS-8 as an independent variable. The optimal cut-off point of SSS-8 was determined by the Youden Index to distinguish the group with severe CSS, and the accuracy of the test was evaluated by its sensitivity and specificity.
All analyses in this study were performed using EZR Version 1.32 [32]. Two-tailed P-values less than 0.05 were considered statistically significant.
Results
Of the 143 participants who met the criteria for this study, 17 were excluded because of missing data or inappropriate responses, leaving the data of 126 available for analysis. The valid response rate was 88.1 percent.
Fifty-one participants were included in the CSS group, defined by a CSI-A of 40 points or higher, and 75 participants were included in the non-CSS group. Table 1 shows a comparison of the data of the two groups: there were more females in the CSS group than in the non-CSS group, the mean age was lower, and more of the patients had a history of CSS. Additionally, the CSS group had significantly higher scores on the HADS anxiety and depression scales, CSI-A, PCS, and SSS-8 than the non-CSS group.
Table 1.
Patient Characteristics (n = 126)
| Non-CSS (n = 75) | CSS (n = 51) | P value | |
|---|---|---|---|
| Sex | < 0.01 | ||
| Male | 34(45.3%) | 9(17.6%) | |
| Female | 41(54.7%) | 42(82.4%) | |
| Age(years) | 60.0 [22.0–81.0] | 49.0 [26.0–83.0] | < 0.01 |
| Education(years) | 14.0 [ 9.0 -20.0] | 14.0 [ 9.0 -16.0] | 0.86 |
| Treatment duration(months) | 36.0 [3.0–204.0] | 66.0 [3.0–204.0] | 0.53 |
| Questionnaire | |||
| CSI-A | 23.0 [1.0–38.0] | 49.0 [40.0–90.0] | < 0.001 |
| CSI-B | 1.0 [ 0.0 -4.0] | 2.0 [ 0.0 -6.0] | < 0.001 |
| SSS-8 | 9.0 [1.0–24.0] | 17.0 [6.0–32.0] | < 0.001 |
| HADS Anxiety | 5.0 [0.0–15.0] | 10.0 [3.0–19.0] | < 0.001 |
| HADS Depression | 5.0 [0.0–15.0] | 9.0 [1.0–17.0] | < 0.001 |
| PCS | 19.0 [0.0–49.0] | 35.0 [0.0–52.0] | < 0.001 |
CSI Central Sensitization Inventory, SSS-8 The Somatic Symptom Scale-8, HAD Hospital Anxiety and Depression Scale, PCS Pain Catastrophizing Scale
Figure 1 shows a comparison of the AUCs for a propensity score including SSS-8 and a propensity score not including SSS-8 for discrimination between the CSS and non-CSS groups. Both AUCs were above 0.7, but the AUC of the propensity score including SSS-8 was significantly larger than that of the propensity score not including SSS-8 (p < 0.05).
Fig. 1.
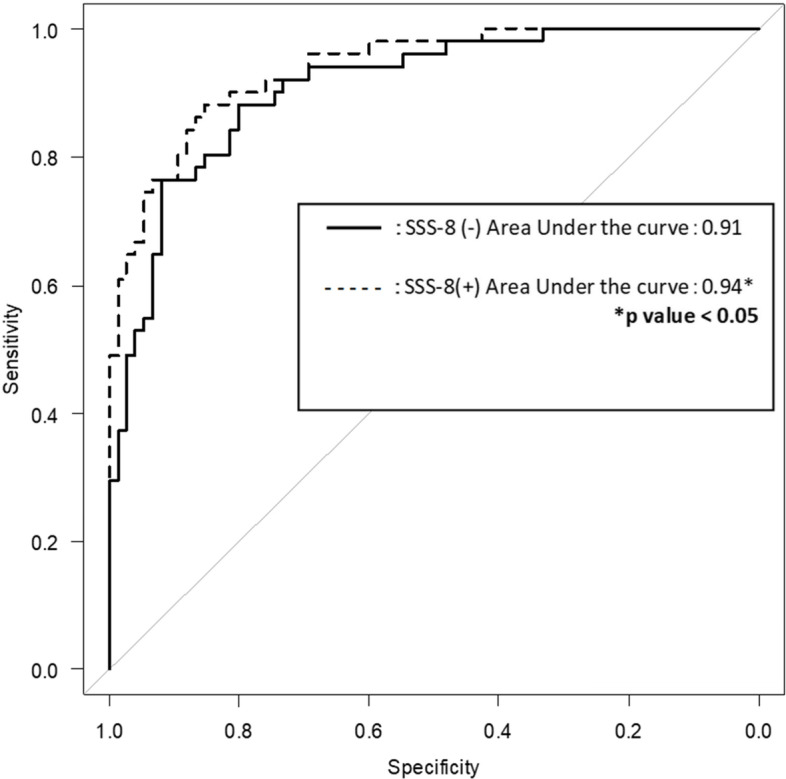
Comparison of discrimination accuracy for central sensitization syndrome between the Somatic Symptom Scale-8 (SSS-8) (+ ; dashed line) and SSS-8 (-; solid line) among the somatic symptom and related disorders patients (n = 126)
Figure 2 shows the ROC curve and cut-off point for the screening patients with severe SSS-8, and Table 2 shows a summary of various cut-off point scores. The optimal SSS-8 cut-off point using the Youden index was 13 points, sensitivity was 84.3 percent, specificity was 77.3 percent, and the AUC was 0.88.
Fig. 2.
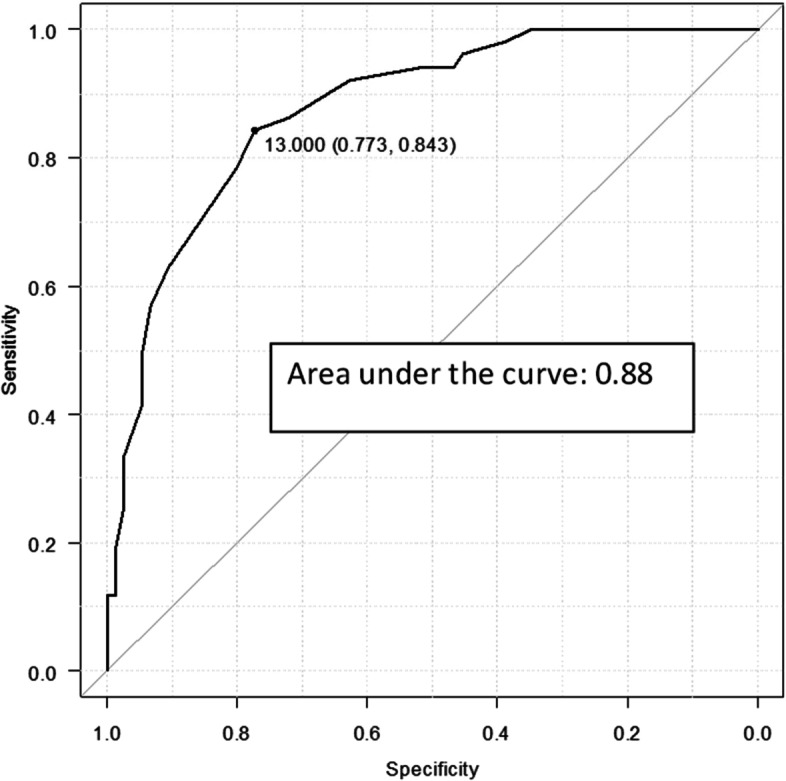
Receiver operating characteristic curve to determine the cut-off points on the Somatic Symptom Scale-8 for discriminating central sensitization syndrome among the somatic symptoms and related disorders patients (n = 126)
Table 2.
Summary of the cut points scores on the Somatic Symptom Scale-8 to discriminate the central sensitization syndrome among the somatic symptoms and related disorders patients (n = 126)
| Cut point score | specificity [95%CI] | sensitivity [95%CI] | positive predictive value [95%CI] | negative predictive value [95%CI] |
|---|---|---|---|---|
| 10 | 0.52 [0.40–0.64] | 0.94 [0.84–0.99] | 0.57 [0.46–0.68] | 0.93 [0.81–0.99] |
| 11 | 0.63 [0.51–0.74] | 0.92 [0.81–0.98] | 0.63 [0.51–0.74] | 0.92 [0.81–0.98] |
| 12 | 0.72 [0.60–0.82] | 0.86 [0.74–0.94] | 0.68 [0.55–0.79] | 0.89 [0.78–0.95] |
| 13 | 0.77 [0.66–0.86] | 0.84 [0.71–0.93] | 0.72 [0.59–0.83] | 0.88 [0.78–0.95] |
| 14 | 0.80 [0.69–0.88] | 0.78 [0.65–0.89] | 0.73 [0.59–0.84] | 0.85 [0.74–0.92] |
| 15 | 0.85 [0.75–0.92] | 0.71 [0.56–0.83] | 0.77 [0.62–0.88] | 0.81 [0.71–0.89] |
| 16 | 0.91 [0.82–0.96] | 0.63 [0.48–0.76] | 0.82 [0.67–0.93] | 0.78 [0.68–0.86] |
| 17 | 0.93 [0.85–0.98] | 0.57 [0.42–0.71] | 0.85 [0.69–0.95] | 0.76 [0.66–0.84] |
CI confidence interval
Discussion
In this study, we examined the utility of the SSS-8 with an optimally chosen cut-off point for discriminating patients with severe disease comorbid with CSS in patients with SSRDs. The SSS-8 was useful for discriminating severity even when confounding factors were considered, and the accuracy of the test was high when the cut-off point was set at 13 or higher.
Generally, sex differences exist in pain sensitivity, and it has been reported that women have lower pain thresholds than men as a biological characteristic [33]. Furthermore, many conditions that fall under CSS are known to be more frequent in women [21, 34–36]. In this sample, there were more women in the CSS group, which is consistent with the characterizations of previous reports. Additionally, the prevalence of CSS conditions tends to decrease with age, for example migraine [37], and the prevalence of irritable bowel syndrome is also low in adults > 50 years of age according to a worldwide meta-analysis [38]. In the present study, the CSS group was younger than the non-CSS group, which is consistent with the trends found in previous studies [37, 38].
The SSS-8 can be used to assist in the diagnosis of somatic symptomatology according to DSM-5 [1] and is useful in assessing clinical severity [39]. A total score of 12 points or higher on the German version of the SSS-8 was considered to be a high somatic symptoms burden on the patient, and scores were divided into five levels of 4 points each [5], yielding three levels of mild to moderate symptoms and two levels of severe cases. In our study, we determined a cut-off value of 13 points on the SSS-8 for discriminating severe cases, which is similar to that in a previous study [5]. The value of the AUC of the propensity score without inclusion of SSS-8 as a variable was 0.91. Therefore, even without using the SSS-8, it may be possible to discriminate severe conditions of SSRDs with high accuracy by just integrating information on background factors such as age, sex, and levels of anxiety, depression, and catastrophic thoughts. However, in our results, the SSS-8 score was found to further improve the accuracy of discriminating severe cases and thus can be a useful tool for screening. Most patients with SSRDs have a high level of functional impairment [40], but no abnormalities are found in biological tests [2]. Therefore, patients often feel anxious about their medically unexplained symptoms and frequently seek explanations from their health care providers [41]. The development of a cut-off point for the SSS-8 will help link the presence or absence of CSS to the intensity of unexplained somatic symptoms, which will provide anxiety relief to patients in the severe group, making it a useful clinical indicator.
Strengths and limitations
This study is clinically meaningful in that it proposes an index for rapid identification of severe symptoms and related disorders. However, there are some limitations to the interpretation. First, we defined the presence of central sensitization syndrome using a questionnaire with CSI. According to previous studies [21, 42], the assessment of central sensitization with the CSI is very precise, but in the present study we did not directly extract physiological changes, and it is unclear how the history of CSS was diagnosed. Second, although the sample size was large enough to reach statistically significant results [43], the sample size was limited by the fact that it was a single site study. Third, the participants may have been better educated than the general SSRDs group [1], and effects of medication and treatment history were not considered. Hence, although our results approximate those of the general population [5], full generalizability cannot be assumed.
Conclusions
In conclusion, this study reported that the SSS-8 was a useful tool for the discrimination of severe cases of SSRDs. We found the optimal cut-off point for this discrimination an SSS-8 score of 13 points.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Authors’ contributions
KH, designed the study protocol and wrote the paper. TT collected and analyzed data and discussed the interpretation of the data. MH collected data and contributed to data interpretation. AK collected data. YN contributed to data interpretation. MH designed the study and contributed to data interpretation. All authors have given consent for publication. The author(s) read and approved the final manuscript.
Funding
This work was supported by grant number 20FC1056 in the MHLW research program on rare and intractable diseases.
Availability of data and materials
We are not able to share our data because sharing data is not permitted by our hospital ethics committees.
Declarations
Ethics approval and consent to participate
This study was approved by the Toho University Medical Center Omori Hospital Ethics Committee, approval number: M20206, with due consideration of the Helsinki Declaration, patient anonymity, and ethics. Written informed consent was obtained from all participants prior to the study.
Consent for publication
All the authors have consented to the publication of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 5th Ed, DSM-5. Washington D. C: American Psychiatric Press; 2013. [Google Scholar]
- 2.Bass C, Peveler R, House A. Somatoform disorders: severe psychiatric illnesses neglected by psychiatrists. Br J Psychiatry. 2001;179:11–14. doi: 10.1192/bjp.179.1.11. [DOI] [PubMed] [Google Scholar]
- 3.Sharpe M. Somatic symptoms: beyond 'medically unexplained'. Br J Psychiatry. 2013;203:320–321. doi: 10.1192/bjp.bp.112.122523. [DOI] [PubMed] [Google Scholar]
- 4.Kroenke K, Spitzer RL, Williams JBW. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Gierk B, Kohlmann S, Kroenke K, Spangenberg L, Zenger M, Brähler E. The somatic symptom scale-8 (SSS-8): a brief measure of somatic symptom burden. JAMA Intern Med. 2014;174:399–407. doi: 10.1001/jamainternmed.2013.12179. [DOI] [PubMed] [Google Scholar]
- 6.Toussaint A, Kroenke K, Baye F, Lourens S. Comparing the Patient Health Questionnaire - 15 and the Somatic Symptom Scale - 8 as measures of somatic symptom burden. J Psychosom Res. 2017;101:44–50. doi: 10.1016/j.jpsychores.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Behm AC, Hüsing P, Löwe B, Toussaint A. Persistence rate of DSM-5 somatic symptom disorder: 4-year follow-up in patients from a psychosomatic outpatient clinic. Compr Psychiatry. 2021;110:152265. doi: 10.1016/j.comppsych.2021.152265. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto K, Takeuchi T, Koyama A, Hiiragi M, Suka S, Hashizume M. Effect of relaxation therapy on benzodiazepine use in patients with medically unexplained symptoms. Biopsychosoc Med. 2020;14:13. doi: 10.1186/s13030-020-00187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn SR. Physical symptoms and physician-experienced difficulty in the physician–patient relationship. Ann Intern Med. 2001;134:897–904. doi: 10.7326/0003-4819-134-9_Part_2-200105011-00014. [DOI] [PubMed] [Google Scholar]
- 10.Sattel H, Lahmann C, Gündel H, Guthrie E, Kruse J, Noll-Hussong M, et al. Brief psychodynamic interpersonal psychotherapy for patients with multisomatoform disorder: randomised controlled trial. Br J Psychiatry. 2012;200:60–67. doi: 10.1192/bjp.bp.111.093526. [DOI] [PubMed] [Google Scholar]
- 11.Budtz-Lilly A, Schroder A, Rask MT, Fink P, Vestergaard M, Rosendal M. Bodily distress syndrome: a new diagnosis for functional disorders in primary care? BMC Fam Pract. 2015;16:180. doi: 10.1186/s12875-015-0393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosendal M, Olde Hartman TC, Aamland A, van der Horst H, Lucassen P, Budtz-Lilly A, et al. "Medically unexplained" symptoms and symptom disorders in primary care: prognosis-based recognition and classification. BMC Fam Pract. 2017;18(1):18. doi: 10.1186/s12875-017-0592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shigetoh H, Tanaka Y, Koga M, Osumi M, Morioka S. The mediating effect of central sensitization on the relation between pain intensity and psychological factors: a cross-sectional study with mediation analysis. Pain Res Manag. 2019;2019(8):3916135. doi: 10.1155/2019/3916135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsudaira K, Kawaguchi M, Murakami M, Fukudo S, Hashizume M, Oka H, et al. Development of a Linguistically Validated Japanese Version of the Somatic Symptom Scale-8 (SSS-8) Jpn Psychosom Med. 2016;56:931–937. [Google Scholar]
- 16.Matsudaira K, Oka H, Kawaguchi M, Murakami M, Fukudo S, Hashizume M, et al. Development of a Japanese version of the somatic symptom Scale-8: psychometric validity and internal consistency. Gen Hosp Psychiatry. 2017;45:7–11. doi: 10.1016/j.genhosppsych.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Mayer TG, Neblett R, Cohen H, Howard KJ, Choi YH, Williams MJ, et al. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012;12:276–285. doi: 10.1111/j.1533-2500.2011.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka K, Nishigami T, Mibu A, Manfuku M, Yono S, Shinohara Y, et al. Validation of the Japanese version of the Central Sensitization Inventory in patients with musculoskeletal disorders. PLoS One. 2017;12:e0188719. doi: 10.1371/journal.pone.0188719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10:556–572. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Zafereo J, Wang-Price S, Kandil E. quantitative sensory testing discriminates central sensitization inventory scores in participants with chronic musculoskeletal pain: an exploratory study. Pain Pract. 2021;21:547–556. doi: 10.1111/papr.12990. [DOI] [PubMed] [Google Scholar]
- 21.Neblett R, Cohen H, Choi Y, Hartzell MM, Williams M, Mayer TG, et al. The central sensitization inventory (CSI): Establishing clinically significant values for identifying central sensitivity syndrome in an outpatient chronic pain sample. J Pain. 2013;14:438–445. doi: 10.1016/j.jpain.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domenech J, Sanchis-Alfonso V, López L, Espejo B. Influence of kinesiophobia and catastrophizing on pain and disability in anterior knee pain patients. Knee Surg Sports Traumatol Arthrosc. 2013;21:1562–1568. doi: 10.1007/s00167-012-2238-5. [DOI] [PubMed] [Google Scholar]
- 23.van Wilgen CP, Vuijk PJ, Kregel J, Voogt L, Meeus M, Descheemaeker F, et al. Psychological distress and widespread pain contribute to the variance of the central sensitization inventory: a cross-sectional study in patients with chronic pain. Pain Pract. 2018;18:239–246. doi: 10.1111/papr.12600. [DOI] [PubMed] [Google Scholar]
- 24.Shigetoh H, Tanaka Y, Koga M, Osumi M, Morioka S. The mediating effect of central sensitization on the relation between pain intensity and psychological factors: a cross-sectional study with mediation analysis. Pain Res Manag. 2019;2019:3916135. doi: 10.1155/2019/3916135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/S0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 27.Herrmann C. International experiences with the Hospital Anxiety and Depression Scale - a review of validation data and clinical results. J Psychosom Res. 1997;42:17–24. doi: 10.1016/S0022-3999(96)00216-4. [DOI] [PubMed] [Google Scholar]
- 28.Hatta H, Higashi A, Yashiro H, Ozasa K, Hayashi K, Kiyota K, et al. A validation of the Hospital Anxiety and Depression Scale. Jpn J Psychosom Med. 1998;38:309–315. [Google Scholar]
- 29.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524–532. doi: 10.1037/1040-3590.7.4.524. [DOI] [Google Scholar]
- 30.Matsuoka H, Sakano Y. Assessment of Cognitive Aspect of Pain; Development, Reliability, and Validation of Japanese Version of Pain Catastrophizing Scale. Jpn J Psychosom Med. 2007;47:95–102. [Google Scholar]
- 31.Zweing MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. doi: 10.1093/clinchem/39.4.561. [DOI] [PubMed] [Google Scholar]
- 32.Kanda Y. Investigation of the freely available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voss U, Lewerenz A, Nieber K. Treatment of irritable bowel syndrome: sex and gender specific aspects. Handb Exp Pharmacol. 2012;214:473–497. doi: 10.1007/978-3-642-30726-3_21. [DOI] [PubMed] [Google Scholar]
- 35.Staud R. The neurobiology of chronic musculoskeletal pain (including chronical regional pain) In: Wallace DJ, Clauw DJ, editors. Fibromyalgia and Other Central Pain Syndromes. Philadelphia, PA: Lippincott Williams and Wilkins; 2005. pp. 45–62. [Google Scholar]
- 36.Shyti R, deVries B, van den Maagdenberg A. Migraine genes and the relation to gender. Headache. 2011;51:880–890. doi: 10.1111/j.1526-4610.2011.01913.x. [DOI] [PubMed] [Google Scholar]
- 37.Bigal ME, Libermann JN, Lipton RB. Age-dependent prevalence and clinical features of migraine. Neurology. 2006;67:246–251. doi: 10.1212/01.wnl.0000225186.76323.69. [DOI] [PubMed] [Google Scholar]
- 38.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 39.Narrow WE, Clarke DE, Kuramoto SJ, Kraemer HC, Kupfer DJ, Greiner L, et al. DSM-5 field trials in the United States and Canada, Part III: development and reliability testing of a cross-cutting symptom assessment for DSM-5. Am J Psychiatry. 2013;170:71–82. doi: 10.1176/appi.ajp.2012.12071000. [DOI] [PubMed] [Google Scholar]
- 40.De Waal MWM, Arnold IA, Eekhof JAH, Van Hemert AM. Somatoform disorders in general practice. Prevalence, functional impairment and comorbidity with anxiety and depressive disorders. Br J Psychiatry. 2004;184:470–476. doi: 10.1192/bjp.184.6.470. [DOI] [PubMed] [Google Scholar]
- 41.Salmon P, Ring A, Humphris GM, Davies JC, Dowrick CF. Primary care consultations about medically unexplained symptoms: how do patients indicate what they want? J Gen Intern Med. 2009;24:450–456. doi: 10.1007/s11606-008-0898-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nijs J, Torres-Cueco R, van Wilgen CP, Girbes EL, Struyf F, Roussel N, et al. Applying modern pain neuroscience in clinical practice: criteria for the classification of central sensitization pain. Pain Physician. 2014;17:447–457. doi: 10.36076/ppj.2014/17/447. [DOI] [PubMed] [Google Scholar]
- 43.Obuchowski NA, Lieber ML, Wians FH., Jr ROC curves in clinical chemistry: uses, misuses, and possible solutions. Clin Chem. 2004;50:1118–1125. doi: 10.1373/clinchem.2004.031823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We are not able to share our data because sharing data is not permitted by our hospital ethics committees.


