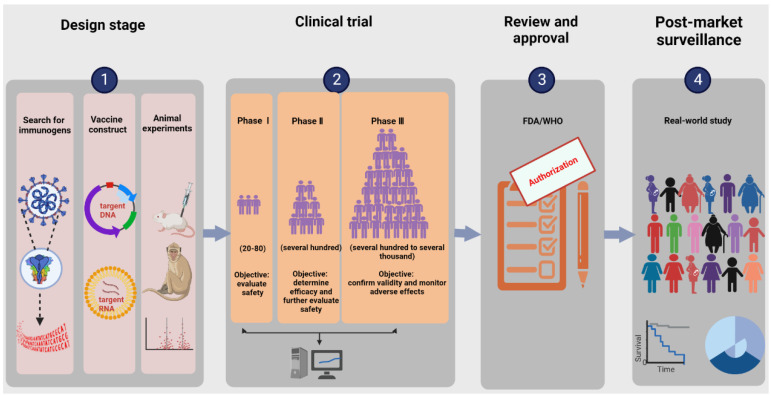
Figure 1.
Illustration of the research and development process for nucleic acid vaccines. The procedure involves an early design stage, clinical trial stage, review and approval stage, and post-market surveillance. The early design stage generally consists of searching for immunogens, designing vaccine structures, and determining toxicological effects and immune effects in animal models. The clinical trials mainly include phases Ⅰ–Ⅲ, targeting primarily practical applications to provide definitive evidence for the safety and efficacy of vaccines.