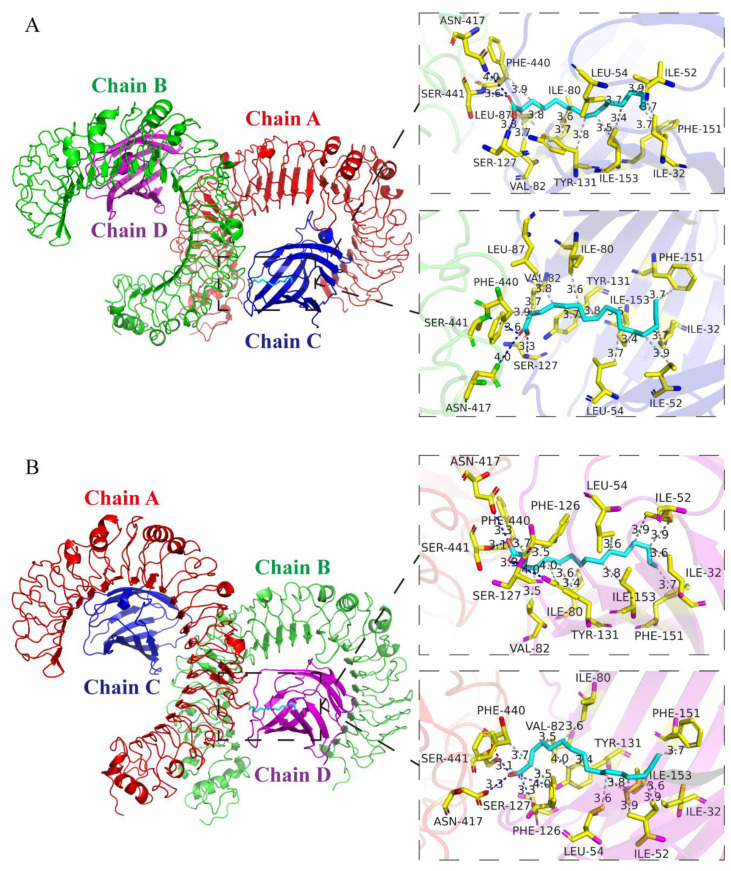
Figure 2.
Molecular docking between PA and TLR4 Four chains of TLR4 are shown as multicolor bands. PA is shown as cyan sticks and TLR4 residues are marked with yellow sticks. (A) The image shows the docking pose, binding sites, and interactions between PA and the C-chain of TLR4. There were three hydrogen bonds between PA and the TLR4 protein residues, which were Ser-127 at 3.3 Å, Asn-417 at 3.6 Å, and Ser-441 at 3.6 Å. In addition, PA and TLR4 also formed 12 hydrophobic interactions in the C-chain, which were Ile-32 at 3.75 Å, Ile-52 at 3.93 Å, Leu-54 at 3.72 Å, Ile-80 at 3.60 Å, Val-82 at 3.73 Å, Leu-87 at 3.77 Å, Tyr-131 at 3.81 Å, Tyr-131 at 3.73 Å, Phe-151 at 3.66 Å, Ile-153 at 3.36 Å, Ile-153 at 3.54 Å, and Phe-440 at 3.88 Å. (B) Docking pose, binding sites, and interactions between PA and D-chain of TLR4. PA formed four hydrogen bonds with the TLR4 protein residues in the binding pocket of the D-chain, which were Ser-127 at 2.33 Å, Tyr-131 at 3.97 Å, Asn-417 at 3.00 Å, and Ser-441 at 2.25 Å. PA and the TLR4 protein residues formed 12 hydrophobic interactions in the binding pocket of the D-chain, which were Ile-32 at 3.57 Å, Ile-52 at 3.87 Å, Ile-52 at 3.94 Å, Ieu-54 at 3.57 Å, Ile-80 at 3.58 Å, Val-82 at 3.53 Å, Phe-126 at 3.5 Å, Tyr-131 at 3.4 Å, Tyr-131 at 3.95 Å, Phe-151 at 3.7 Å, Ile-153 at 3.8 Å, and Phe-440 at 3.73 Å.