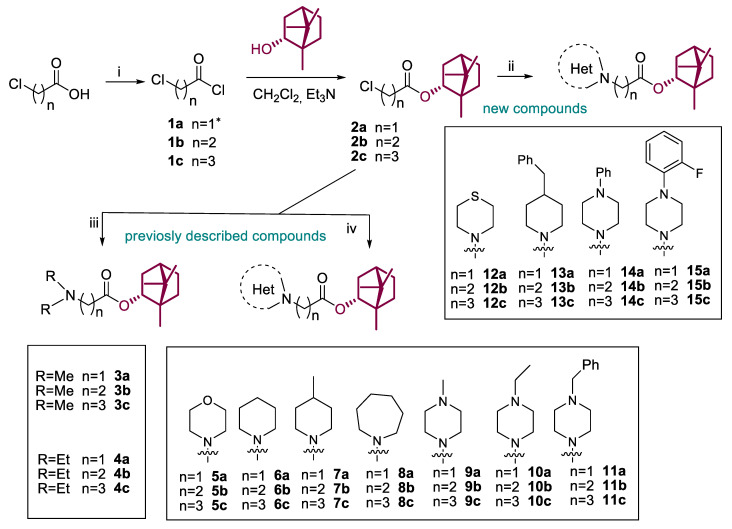
Scheme 1.
Reagents and conditions: (i) (COCl)2 (3 equiv), CH2Cl2, DMF (one drop), stirring at room temperature for 4 h; (ii) the corresponding amine (1.2 equiv), K2CO3 (4 equiv), KI (1 equiv), CH3CN, room temperature or slight heating for 12–48 h, yields 32–91%; (iii) excess of dimethylamine (40% wt. solution in ethanol) or diethylamine, K2CO3 (2 equiv), CH3CN, room temperature or reflux for 12 h, yields 35–53%; (iv) the corresponding amine (1.5 equiv), Et3N (1 equiv), CH2Cl2, room temperature or slight heating for 12–48 h, yields 32–91%. * Compound 1a was used as a commercial reagent.