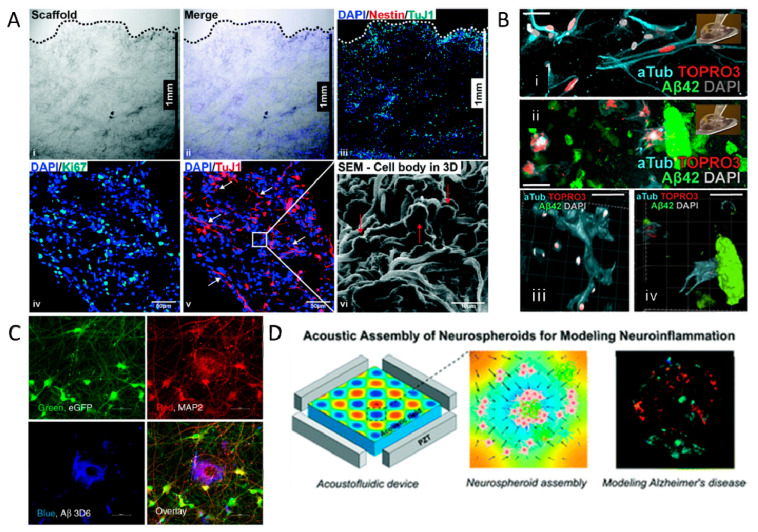
Figure 2.
3D in vitro models of Alzheimer’s disease. (A) PLGA scaffold encapsulating iPSC-derived NPCs, shown by proliferation marker Ki67, undifferentiated cell marker Nestin, and neural differentiation marker TuJ1. (i–iii) Cross-section ofthe 3D microfiber scaffold showing cell infiltration, distribution and differentiation. (iv,v) cell proliferation and differentiation. (vi) SEM image of the scaffold cross-section. Reprinted with permission from [134]. Copyright 2020, The Royal Society of Chemistry. (B) Reduction of neural plasticity in neural stem cells as a result of Aβ plaques. Image (i,iii) indicate healthy neural stem cells, which can form new synaptic connections, shown in (iii). Image (ii,iv) have the Aβ plaques incorporated in the hydrogel and are unable to form new synaptic connections. Reprinted with permission from [135]. Copyright 2018, Elsevier. (C) 3D spheroids derived from human neural progenitor cells, displaying Aβ pathology (shown in blue). Green and Red channels depict the neuronal cells and differentiated neurons, respectively. Reprinted from [138]. Copyright 2020, Nature Portfolio. (D) Acoustofluidic platform to assemble cells and Aβ oligomers into neuro-spheroids representing AD. Reproduced with permission of The Royal Society of Chemistry [139]. (i) cross-section of the 3D microfiber scaffold after sectioning (dotted line indicates the top surface of the 3D scaffold); (ii,iii) cell infiltration, distribution and differentiation of iPSC-derived NPCs (8529 cell line) inside the 3D scaffold as assessed via staining for TuJ1 (green) and Nestin (red) markers on D13; (iv) cell proliferation assessed by the Ki67 (green) marker and (v) cell differentiation with neurite formation indicated by the TuJ1 (red) marker of hESC-derived NPCs on D13; nuclei were counterstained with DAPI (blue); (vi) SEM image of the scaffold cross-section showing cell morphology and attachment on microfibers at 2000× magnification.