Abstract
The aim of this systematic review and meta-analysis was to evaluate the association between glycemic control (HbA1c) and functional capacity (VO2max) in individuals with type 1 diabetes (T1DM). A systematic literature search was conducted in EMBASE, PubMed, Cochrane Central Register of Controlled Trials, and ISI Web of Knowledge for publications from January 1950 until July 2020. Randomized and observational controlled trials with a minimum number of three participants were included if cardio-pulmonary exercise tests to determine VO2max and HbA1c measurement has been performed. Pooled mean values were estimated for VO2max and HbA1c and weighted Pearson correlation and meta-regression were performed to assess the association between these parameters. We included 187 studies with a total of 3278 individuals with T1DM. The pooled mean HbA1c value was 8.1% (95%CI; 7.9–8.3%), and relative VO2max was 38.5 mL/min/kg (37.3–39.6). The pooled mean VO2max was significantly lower (36.9 vs. 40.7, p = 0.001) in studies reporting a mean HbA1c > 7.5% compared to studies with a mean HbA1c ≤ 7.5%. Weighted Pearson correlation coefficient was r = −0.19 (p < 0.001) between VO2max and HbA1c. Meta-regression adjusted for age and sex showed a significant decrease of −0.94 mL/min/kg in VO2max per HbA1c increase of 1% (p = 0.024). In conclusion, we were able to determine a statistically significant correlation between HbA1c and VO2max in individuals with T1DM. However, as the correlation was only weak, the association of HbA1c and VO2max might not be of clinical relevance in individuals with T1DM.
Keywords: type 1 diabetes, HbA1c, VO2max, systematic review, meta-analysis
1. Introduction
Regular physical activity (PA) represents a highly relevant non-pharmaceutical-glucose lowering activity in people with type 1 diabetes (T1DM) and is recommended to be conducted by means of moderate-to-vigorous intensity aerobic exercise for 150 min at least three days a week in most adults with T1DM [1]. Despite the challenge of PA potentially contributing to exercise-induced dysglycemia in T1DM, potential benefits have been demonstrated in regard to improving cardio-respiratory fitness, cardiovascular risk factors, and quality of life [2]. Besides the impact of PA on these aforementioned parameters, deteriorated glycemic control may act as an antagonist, which in T1DM remains a matter of debate [3] In people with type 2 diabetes (T2DM), research evidence suggests an inverse correlation between cardiorespiratory fitness assessed by maximum oxygen uptake (VO2max) and clinical markers of glycemic control (high HbA1c, high fasting glucose) also after adjustment for age, body weight and markers of adiposity [4]. It has been speculated that VO2max as a determinant for PA may be positively associated with β-cell compensation mechanisms such as improvements in glucolipotoxicity [5]. This may lead to lower levels of pro-inflammatory cytokines and increased secretion of various growth factors and hormones which may contribute to higher β-cell mass [5]. However, these suggested mechanisms in T2DM are hardly transferable to a T1DM population that often has completely lost their endogenous insulin production during the progress of the autoimmune disease.
We have recently shown that individuals with early-stage T1DM compared to healthy controls are characterized by a lower maximum oxygen uptake including decreased absolute and relative oxygen uptake (VO2) as well as weaker oxygen reserves and oxygen pulse when determined during a cardiopulmonary exercise (CPX) test on a cycle ergometer [6]. Interestingly, these altered responses were not associated with HbA1c but this might have been due to the tight glycemic control in the T1DM cohort of this study (mean HbA1c 6.9% [6.2;7.7%]). Thus, the jury is still out whether or not glucose control assessed by HbA1c is associated with maximum oxygen uptake.
In clinical practice, this question is highly relevant for physically active patients and in particular for competitive athletes with T1DM. Therefore, this systematic review investigates the association between HbA1c levels and VO2max assessed during CPX testing in individuals with T1DM.
2. Materials and Methods
This systematic review and comprehensive analysis were conducted according to the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines. The study was registered at the International Prospective Register of Systematic Reviews (Prospero) prior to initiation of the literature search (CRD42020141164).
2.1. Data Sources and Study Selection
The following electronic libraries were searched to identify relevant publications: EMBASE, PubMed, Cochrane Central Register of Controlled Trials, and ISI Web of Knowledge. Studies from January 1950 until July 2020 were included in the analysis. Search items were included as shown in Supplemental Tables S1–S4. Randomized and observational studies with a minimum number of three participants were included if HbA1c measurements and CPX tests to determine VO2max had been performed. Only published studies were considered. Moreover, no in silico or animal studies were included. Moreover, systematic reviews and meta-analysis were excluded. Additionally, duplicates of articles were discarded. Study titles and abstracts were reviewed including relevant studies fulfilling the inclusion criteria. Then, the full text of these studies was digitally saved and read by two independent authors (MLE, F.J.R.D.). Another independent author (O.M) monitored the identified studies and solved potential disagreements. The SD values were converted to standard error (SE = SD/√n) [7]. Interquartile range (IQR) or confidence intervals were converted using the formula provided in the supplementary file. If studies included more than one appropriate data set, these data were extracted and analyzed separately.
2.2. Criteria for Inclusion in the Review
The following criteria had to be met for the study abstract to be considered eligible for manuscript data extraction: (a) reporting of an association between HbA1c at baseline and VO2max, (b) at least three participating children aged 6–12, adolescents aged 13 to 17 or adults older than 18 years, (c) participants with type 1 diabetes, (d) observational, cross-sectional or randomized controlled study design, (e) conducted a CPX test. Studies without clear specification of diabetes type were not included.
2.3. Data Extraction and Quality Assessment
Following information, if available, was extracted from all studies: authors, year of publication, country of study origin, trial design, sample size, age and sex of participants and CPX-test procedure. If data were missing, authors were asked to provide these data. If the main outcome (e.g., BG delta) was not reported, could not be retrieved after contacting the authors, or computed, the study was excluded.
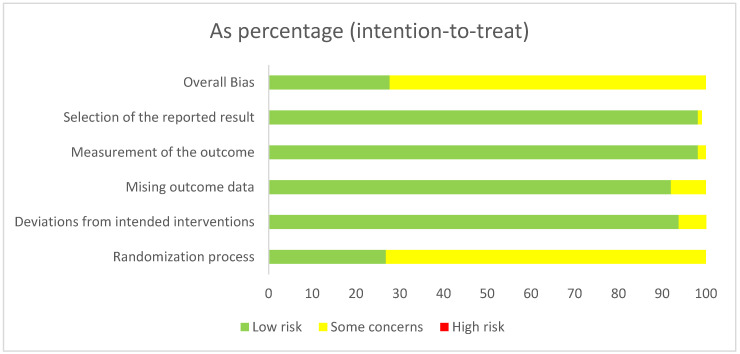
Studies were independently assessed by two investigators (M.L.E., F.J.R.D.) for methodological quality using the risk of bias assessment tool from the Cochrane Collaboration [8] in its revised version [9]. The following sources of bias were detected: overall bias, selection of the reported result, bias of the measurement of the outcome, missing outcome data, deviations from the intended interventions and randomization process (Figure 1). We did not exclude any studies based on the risk of bias assessment since the included trials were judged to possess a low risk of bias following the assessment (Figure 1).
Figure 1.
Risk of bias summary. Risk of bias was assessed according to the methods recommended by the Cochrane Collaboration [8].
2.4. Data Synthesis and Analysis
A narrative descriptive analysis was performed to summarize the characteristics of studies, such as population, age and type of CPX testing. VO2max was defined as the maximum oxygen consumption given in each study measured via a CPX test on a spirometric device independent of manufacturing company. HbA1c values were recorded as given in the anthropometry section of the included cohorts within each manuscript. Studies were excluded if VO2max values were not measured according to the guidelines of the American College of Sports Medicine [10,11].
Meta-Analysis
The meta-analysis was performed using the random effects model and Hedges’ g method as a number of studies only had a small sample size. The effect size (delta BG) was summarized and presented as the pooled mean with corresponding 95% confidence interval (CI). The negative pooled mean indicated a higher decrease in BG following physical exercise. The heterogeneity in the effect size was assessed by estimating I2 statistics and Cochran’s Q test for homogeneity. The difference in effect size with respect to cycling versus running studies and other study-level categorical covariates was assessed by performing sub-group analysis of the effect size for each covariate and group differences in the effect size were assessed via Cochran’s Q test for homogeneity. The difference in effect size with respect to the study-level continuous covariates was assessed by meta-regression analysis. Furthermore, simple and multiple meta-regression were performed to assess the crude and adjusted association of each study-level covariate with the effect size within the strata of cycling and treadmill studies. The results of the meta-regression were reported as a coefficient with corresponding 95% CI and p values. Publication bias was assessed in terms of meta-bias using Egger’s test and visualized via a funnel plot. The results of the meta-analysis are presented in the supplementary material.
3. Results
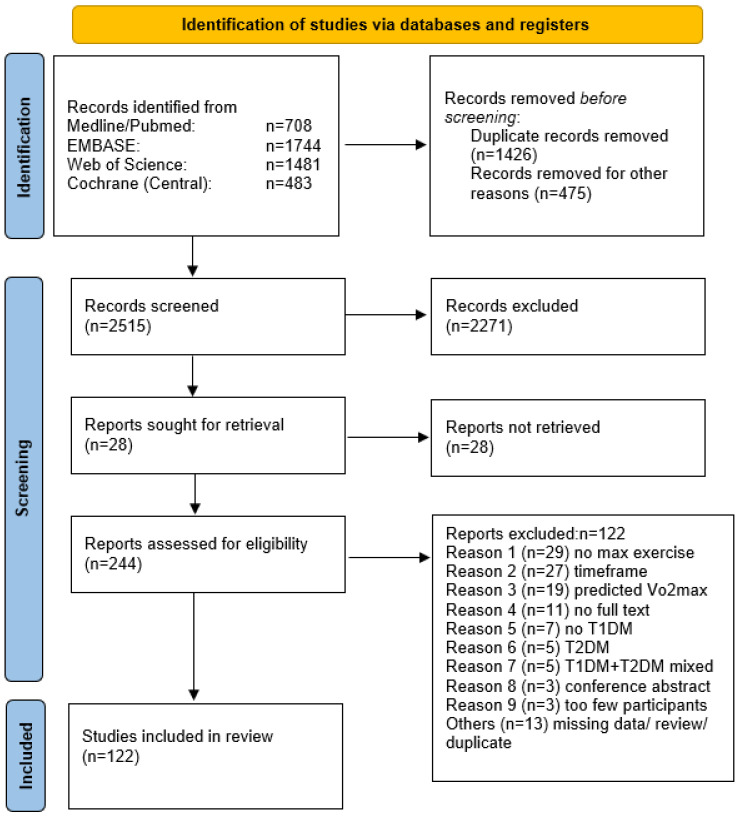
A total of 122 studies were extracted from the initially screened 4416 titles. The steps of the article selection process are described as a flow diagram in Figure 2. Studies published between 1989 and 2020 included data from 3278 participants with T1DM. Due to the crossover designs of the studies the results were split for analysis and led to a total number of 187 study results included. We included 57 case–control studies, 41 randomized controlled trials, 2 secondary outcome analyses from randomized controlled trials, 7 non-randomized comparative studies and 15 cohort studies. Groups were separated by adolescents, adolescents/adults and adults, since some study cohorts did not include both age-groups without a clear separation within the results of the study. Detailed information is shown in Figure 2.
Figure 2.
PRISMA statement [12].
All study-specific outcomes included in this systematic review and meta-analysis are shown in Table 1.
Table 1.
Studies included in systematic review and meta-analysis with HbA1c and VO2max.
| Title | Sex | Age-Set | Type of Exercise | HbA1c ± SD (%) | VO2max/peak ± SD (mL/kg/min) | Sample Size |
|---|---|---|---|---|---|---|
| Abraham, MB 2017 [13] | both (m/w = 4/4) | adolescents | other | 7.8 ± 1 | 39.7 ± 6.10 | 8 |
| Adolfsson, P 2012 (a) [14] | male | adolescents | cycle ergometry | 7.6 ± 0.88 | 56.00 ± 5.50 | 12 |
| Adolfsson, P 2012 (b) | female | adolescents | cycle ergometry | 8.1 ± 0.58 | 44.00 ± 6.68 | 12 |
| Adolfsson, P 2012 (c) | both | adolescents | cycle ergometry | 7.9 ± 3.07 | 49.80 ± 9.90 | 12 |
| Al Khalifah, RA (a) 2016 [15] | both (m/w = 15/8) | adults | cycle ergometry/treadmill | 7.8 ± 0.9 | 44.00 ± 8.80 | 23 |
| Al Khalifah, RA (b) 2016 | both (m/w = 11/10) | adults | cycle ergometry/treadmill | 7.6 ± 0.9 | 26.70 ± 5.00 | 21 |
| Atalay, M 1997 [16] | male | adults | cycle ergometry | 7.3 ± 1.7 | 46.00 ± 6.90 | 9 |
| Austin, A 1993 [17] | both (m/w = 28/31) | adolescents | cycle ergometry | 10.6 ± 2.1 | 33.70 ± 7.00 | 59 |
| Bak, JF 1989 [18] | undefined | adults | cycle ergometry | 7.9 ± 1.4 | 45.70 ± 7.40 | 7 |
| Baldi, JC 2010 [19] | both (m/w = 80%/20%) | adults | cycle ergometry | 7.3 ± 0.8 | 42.00 ± 8.00 | 12 |
| Bally, L (1) 2016 [20] | male | adults | cycle ergometry | 7 ± 0.6 | 47.90 ± 10.20 | 12 |
| Bally, L (2) 2016 [21] | male | adults | cycle ergometry | 7 ± 0.6 | 48.00 ± 11.20 | 10 |
| Baraldi, E 1992 [22] | both (m/w = 17/16) | adolescents | treadmill | 8.9 ± 1.8 | 41.20 ± 5.90 | 33 |
| Benbassat, CA 2001 [23] | both (m/w = 9/7) | adults | cycle ergometry | 8.6 ± 1.8 | 27.50 ± 12.00 | 15 |
| Bjornstad, P 2018 (a) [24] | both (m/w = 48%/52%) | adolescents | cycle ergometry | 9 ± 1.6 | 25.80 ± 4.60 | 27 |
| Bjornstad, P 2018 (b) | both (m/w = 48%/52%) | adolescents | cycle ergometry | 8.8 ± 1.4 | 33.00 ± 7.80 | 48 |
| Bjornstad, P 2018 (c) | both (m/w = 48%/52%) | adolescents | cycle ergometry | 8.2 ± 1.4 | 33.20 ± 4.40 | 52 |
| Bjornstad, P 2018 (d) | both (m/w = 48%/52%) | adolescents | cycle ergometry | 9 ± 1.6 | 22.80 ± 4.90 | 27 |
| Bjornstad, P 2018 (e) | both (m/w = 48%/52%) | adolescents | cycle ergometry | 8.8 ± 1.4 | 23.00 ± 5.80 | 48 |
| Bjornstad, P 2018 (f) | both (m/w = 48%/52%) | adolescents | cycle ergometry | 8.2 ±1.4 | 30.10 ± 8.00 | 52 |
| Bjornstad, P 2015 [25] | both | adolescents | cycle ergometry | 8.5 ± 1.4 | 31.50 ± 6.30 | 69 |
| Boff, W 2019 (a) bl. [26] | both (m/w = 3/6) | adults | cycle ergometry | 8.2 ± 1.3 | 34.00 ± 6.30 | 9 |
| Boff, W 2019 (b) pi. | both (m/w = 3/6) | adults | cycle ergometry | 8.2 ± 1.3 | 40.10 ± 4.30 | 9 |
| Boff, W 2019 (c) bl. | both (m/w = 5/4) | adults | cycle ergometry | 8.4 ± 0.9 | 33.00 ± 8.20 | 9 |
| Boff, W 2019 (d) pi. | both (m/w = 5/4) | adults | cycle ergometry | 8.4 ± 0.9 | 36.00 ± 8.80 | 9 |
| Boff, W 2019 (e) bl. | both (m/w = 4/5) | adults | cycle ergometry | 8.8 ± 2.3 | 33.20 ± 10.00 | 9 |
| Boff, W 2019 (f) pi. | both (m/w = 4/5) | adults | cycle ergometry | 8.8 ± 2.3 | 32.70 ± 10.00 | 9 |
| Bracken, RM 2012 [27] | both (m/w = 2/5) | adults | treadmill | 9.16 ± 2.74 | 38.90 ± 4.40 | 7 |
| Bracken, RM 2011 [28] | both (m/w = 6/1) | adults | treadmill | 8.3 ± 0.1 | 43.50 ± 0.90 | 7 |
| Brazeau, AS (1a) 2012 [29] | male | adults | cycle ergometry | 7.71 ± 1.25 | 35.90 ± 10.50 | 22 |
| Brazeau, AS (1b) 2012 | male | adults | cycle ergometry | 7.42 ± 1.25 | 29.90 ± 7.70 | 18 |
| Brazeau, AS (1c) 2012 | female | adults | cycle ergometry | 7.71 ± 1.25 | 28.30 ± 6.20 | 15 |
| Brazeau, AS (1d) 2012 | female | adults | cycle ergometry | 7.42 ± 1.25 | 22.40 ± 5.20 | 20 |
| Brazeau, AS (2a) 2012 [30] | male | adults | cycle ergometry | 7.5 ± 0.9 | 33.10 ± 9.80 | 40 |
| Brazeau, AS (2b) 2012 | female | adults | cycle ergometry | 7.7 ± 1.6 | 24.80 ± 6.30 | 37 |
| Brazeau, AS (2c) 2012 | both | adults | cycle ergometry | 7.6 ± 1.3 | 29.20 ± 9.20 | 77 |
| Brugnara, L 2012 [31] | male | adults | cycle ergometry | 6.9 ± 1 | 35.00 ± 6.50 | 10 |
| Bussau, VA 2006 [32] | male | adults | cycle | 7.4 ± 0.8 | 44.50 ± 4.20 | 7 |
| Bussau, VA 2007 [33] | male | adults | cycle ergometry | 7.4 ± 0.7 | 45.20 ± 5.00 | 7 |
| Campaigne, BN 1987 [34] | male | adults | cycle ergometry | 7.4 ± 0.3 | 36.60 ± 1.60 | 9 |
| Campbell, MD 2013 [35] | male | adults | treadmill | 7.7 ± 0.3 | 53.00 ± 1.00 | 11 |
| Campbell, MD (1) 2014 [36] | male | adults | treadmill | 7.7 ± 0.4 | 54.00 ± 1.00 | 8 |
| Campbell, MD (2) 2014 [37] | male | adults | treadmill | 6.7 ± 0.7 | 52.00 ± 4.00 | 10 |
| Campbell, MD (1) 2015 [38] | both (m/w = 7/2) | adults | treadmill | 8.1 ± 0.2 | 41.80 ± 1.60 | 9 |
| Campbell, MD (2) 2015 [39] | male | adults | treadmill | 6.9 ± 0.2 | 51.30 ± 2.10 | 10 |
| Chokkalingam, K 2007 [40] | male | adults | cycle ergometry | 7.9 ± 0.2 | 44.50 ± 1.20 | 8 |
| de Jesus, IC 2019 [41] | both (m/w = 5/4) | adolescents | cycle ergometry | 9.39 ± 1.25 | 38.79 ± 10.02 | 9 |
| de Lima, VA 2017 [42] | both (m/w = 25/20) | adolescents | cycle ergometry | 9.15 ± 1.61 | 38.38 ± 7.54 | 45 |
| D’hooge, R (a) 2011 bl. [43] | both | adolescents | cycle ergometry | 8.13 ± 1.02 | 32.87 ± 7.83 | 8 |
| D’hooge, R (b) 2011 pi. | both | adolescents | cycle ergometry | 8.08 ± 0.97 | 32.99 ± 9.58 | 8 |
| D’hooge, R (c) 2011 bl. | both | adolescents | cycle ergometry | 8.55 ± 0.82 | 35.19 ± 6.71 | 8 |
| D’hooge, R (d) 2011 pi. | both | adolescents | cycle ergometry | 8.48 ± 0.9 | 34.69 ± 9.24 | 8 |
| Dovc, K (a) 2017 [44] | male | adolescents | cycle ergometry | 7.5 ± 0.5 | 49.2 ± 8.1 | 11 |
| Dovc, K (b) 2017 | female | adolescents | cycle ergometry | 7.9 ± 0.7 | 36.1 ± 4 | 9 |
| Dovc, K (c) 2017 | both | adolescents | cycle ergometry | 7.7 ± 0.6 | 43.3 ± 9.3 | 20 |
| Ebeling, P (a) 1995 [45] | undefined | adults | cycle ergometry | 8.4 ± 0.4 | 52.0 ± 1.0 | 11 |
| Ebeling, P (b) 1995 | undefined | adults | cycle ergometry | 7.2 ± 0.2 | 42.0 ± 1.0 | 12 |
| Farinha, JB (a) 2018 bl. [46] | both (m/w = 5/4) | adults | cycle ergometry | 7.5 ± 1.5 | 31.3 ± 6.0 | 9 |
| Farinha, JB (b) 2018 pi. | both (m/w = 5/4) | adults | cycle ergometry | 7.2 ± 1.1 | 37.4 ± 8.7 | 9 |
| Farinha, JB (c) 2018 bl. | both (m/w = 5/4) | adults | cycle ergometry | 8.1 ± 1.3 | 32.4 ± 6.3 | 9 |
| Farinha, JB (d) 2018 pi. | both (m/w = 5/4) | adults | cycle ergometry | 8 ± 0.8 | 34.3 ± 5.2 | 9 |
| Farinha, JB (e) 2018 bl. | both (m/w = 5/5) | adults | cycle ergometry | 7.5 ± 1 | 31.4 ± 7.1 | 10 |
| Farinha, JB (f) 2018 pi. | both (m/w = 5/5) | adults | cycle ergometry | 7.2 ± 0.7 | 33.0 ± 7.8 | 10 |
| Faulkner, MS (a) 2010 bl. [47] | both (m/w = 9/3) | adolescents | cycle ergometry | 9.4 ± 1.8 | 33.3 ± 6.9 | 12 |
| Faulkner, MS (b) 2010 pi. | both (m/w = 9/3) | adolescents | cycle ergometry | 9.4 ± 2.1 | 35.8 ± 8.8 | 12 |
| Faulkner, MS 2005 [48] | both (m/w = 57/48) | adolescents | cycle ergometry | 8.7 ± 1.6 | 34.4 ± 8.8 | 105 |
| Fintini, D 2012 [49] | both (m/w = 15/20) | children | treadmill | 7.7 ± 0.8 | 36.2 ± 7.4 | 35 |
| Franc, S 2015 [50] | both (m/w = 11/9) | adults | cycle ergometry | 7.9 ± 0.9 | 33.0 ± 10.0 | 20 |
| Francis, SL (a) 2015 [51] | male | adolescents | treadmill | 8.6 ± 0.9 | 50.0 ± 6.5 | 10 |
| Francis, SL (b) 2015 | female | adolescents | treadmill | 8.2 ± 0.9 | 44.0 ± 6.3 | 10 |
| Francis, SL (c) 2015 | both | adolescents | treadmill | 8.4 ± 0.7 | 47.0 ± 6.9 | 20 |
| Fuchsjager-Mayrl, G 2002 (a) bl. [52] | both (m/w = 7/11) | adults | cycle ergometry | 7.3 ± 0.2 | 28.1 ± 1.2 | 18 |
| Fuchsjager-Mayrl, G 2002 (b) pi.(1) | both (m/w = 7/11) | adults | cycle ergometry | 7.7 ± 0.3 | 31.8 ± 2 | 18 |
| Fuchsjager-Mayrl, G 2002 (c) pi.(2) | both | adults | cycle ergometry | 7.5 ± 0.3 | 35.7 ± 2.8 | 15 |
| Fuchsjager-Mayrl, G 2002 (d) pi.(3) | both | adults | cycle ergometry | 7 ± 0.2 | 28.4 ± 1.8 | 13 |
| Fuchsjager-Mayrl, G 2002 (e) bl. | both | adults | cycle ergometry | 7.4 ± 0.4 | 29.6 ± 2.3 | 8 |
| Fuchsjager-Mayrl, G 2002 (f) pi. | both | adults | cycle ergometry | 7.4 ± 0.2 | 29.7 ± 2.4 | 8 |
| Giani, E 2018 [53] | both (m/w = 53%/47%) | adolescents | cycle ergometry | 7.4 ± 1.0 | 33.2 ± 6.2 | 17 |
| Goulding, R 2020 [54] | male | adults | cycle ergometry | 7.3 ± 0.9 | 36.4 ± 4.7 | 17 |
| Gray, BJ 2016 [55] | both (m/w = 2/5) | adults | treadmill | 9.2 ± 0.6 | 38.9 ± 4.4 | 7 |
| Guelfi, KJ 2005 [56] | both (m/w = 4/3) | adults | other | 7.4 ± 1.5 | 39.4 ± 7.4 | 7 |
| Guelfi, KJ 2007 [57] | both (m/w = 5/4) | adults | cycle ergometry | 7.7 ± 0.8 | 41.8 ± 4.6 | 9 |
| Gusso, S 2008 [58] | female | adolescents | cycle ergometry | 8.8 ± 0.3 | 31.6 ± 2 | 12 |
| Gusso, S 2012 [59] | both (m/w = 27/26) | adolescents | cycle ergometry | 8.7 ± 0.2 | 33.1 ± 1.0 | 53 |
| Haagglund, H 2012 [60] | male | adults | cycle ergometry | 7.7 ± 0.9 | 36 ± 4 | 10 |
| Heise, T 2016 [61] | both (m/w = 35/5) | adults | other | 7.7 ± 0.8 | 39.4 ± 3.7 | 40 |
| Heyman, E 2020 [62] | both (m/w = 12/4) | adults | cycle ergometry | 8.3 ± 1.5 | 34.9 ± 7.2 | 16 |
| Heyman, E 2007 [63] | female | adolescents | cycle ergometry | 8.1 ± 1.3 | 30.6 ± 4.0 | 19 |
| Hilberg, T 2004 [64] | male | adults | cycle ergometry | 7.2 ± 0.2 | 49.0 ± 2.2 | 16 |
| Jenni, S 2008 [65] | male | adults | cycle ergometry | 6.7 ± 0.2 | 50.3 ± 4.5 | 7 |
| Jensen, T 1988 (a) [66] | both (m/w = 6/4) | adults | cycle ergometry | 7.5 ± 0.95 | 40.7 ± 9.5 | 10 |
| Jensen, T 1988 (b) | both (m/w = 6/4) | adults | cycle ergometry | 8.7 ± 1.21 | 28.4 ± 8.8 | 10 |
| Jensen, T 1988 (c) | both (m/w = 6/4) | adults | cycle ergometry | 9.3 ± 1.0 | 28.2 ± 4.2 | 10 |
| Komatsu, WR 2010 (a) [67] | undefined | adults | treadmill | 7.5 ± 6.2 | 42.4 ± 5.5 | 15 |
| Komatsu, WR 2010 (b) | undefined | adults | treadmill | 9 ± 1.3 | 34.8 ± 3.3 | 12 |
| Komatsu, WR 2005 [68] | both (m/w = 38/34) | adolescents | treadmill | 8.1 ± 2.2 | 41.6 ± 7.7 | 72 |
| Koponen, AS 2013 [69] | male | adults | cycle ergometry | 7.65 ± 0.8 | 35.4 ± 4.8 | 12 |
| Kornhauser, C 2012 [70] | both (m/w = 5/5) | adolescents | treadmill | 10 ± 1.0 | 37.5 ± 2.7 | 10 |
| Laaksonen, DE 2000 (a) bl. [71] | male | adults | cycle ergometry | 8.2 ± 1.1 | 43.4 ± 8.0 | 20 |
| Laaksonen, DE 2000 (b) pi. | male | adults | cycle ergometry | 8 ± 1.0 | 46.1 ± 6.6 | 20 |
| Laaksonen, DE 1996 [72] | male | adults | cycle ergometry | 7.3 ± 1.7 | 46 ± 6.9 | 9 |
| Landt, KW 1985 (a) bl. [73] | both (m/w = 3/6) | adolescents | cycle ergometry | 12 ± 1.0 | 36.3 ± 3 | 9 |
| Landt, KW 1985 (b) pi. | both (m/w = 3/6) | adolescents | cycle ergometry | 12 ± 1.0 | 39.3 ± 3 | 9 |
| Landt, KW 1985 (c) bl. | both (m/w = 4/2) | adolescents | cycle ergometry | 12 ± 1.0 | 39.2 ± 3.4 | 6 |
| Landt, KW 1985 (d) pi. | both (m/w = 4/2) | adolescents | cycle ergometry | 12 ± 1.0 | 37.5 ± 3.3 | 6 |
| Lee, MJ 2016 [74] | both (m/w = 45.8%/54.2%) | (adolescents)/adults | cycle ergometry | 7.9 ± 1.3 | 34.9 ± 5.8 | 24 |
| Lehmann, R 1997 (a) bl. [75] | both (m/w = 13/7) | adults | cycle ergometry | 7.6 ± 1.0 | 41.2 ± 13.1 | 20 |
| Lehmann, R 1997 (b) pi. | both (m/w = 13/7) | adults | cycle ergometry | 7.5 ± 0.9 | 45.0 ± 13.2 | 20 |
| Matthys, D 1996 [76] | both (m/w = 12/18) | adolescents | cycle ergometry | 10 ± 0.3 | 32.5 ± 2.1 | 30 |
| McCarthy, O 2020 [77] | male | adults | cycle ergometry | 6.8 ± 0.6 | 73.1 ± 3.8 | 16 |
| McKewen, MW 1999 [78] | male | adults | cycle ergometry | 7.2 ± 1.2 | 50.3 ± 7.4 | 7 |
| Michaliszyn, SF 2009 [79] | both (m/w = 60/49) | adolescents | other | 8.7 ± 1.6 | 34.7 ± 8.9 | 109 |
| Moser, O 2017 [80] | both (m/w = 51/13) | adults | cycle ergometry | 7.8 ± 1.0 | 37.0 ± 5.0 | 64 |
| Moser, O 2019 [81] | both (m/w = 5/4) | adults | cycle ergometry | 7.2 ± 0.6 | 39.0 ± 12.0 | 9 |
| Moser, O 2018 (1) [82] | male | adults | cycle ergometry | 7.4 ± 0.6 | 52.5 ± 6.6 | 7 |
| Moser, O 2018 (2) [83] | both (m/w = 51/13) | adults | cycle ergometry | 7.8 ± 1.0 | 37.0 ± 5.0 | 64 |
| Murray, FT 1988 [84] | male | adults | other | 12 ± 0.6 | 33.5 ± 2.6 | 8 |
| Nadeau, KJ 2010 [85] | both (m/w = 6/6) | adolescents | cycle ergometry | 8.65 ± 1.6 | 31.5 ± 7.6 | 12 |
| Nguyen, T 2015 (a) [86] | both (m/w = 5/3) | adolescents | cycle ergometry | 7.4 ± 0.5 | 38.5 ± 5.8 | 8 |
| Nguyen, T 2015 (b) | both (m/w = 5/3) | adolescents | cycle ergometry | 11.1 ± 1.0 | 33.2 ± 5.6 | 8 |
| Niranjan, V 1997 (a) [87] | both (m/w = 7/2) | adults | cycle ergometry | 5.6 ± 0.2 | 26.9 ± 2.6 | 9 |
| Niranjan, V 1997 (b) | both (m/w = 4/5) | adults | cycle ergometry | 8.8 ± 0.5 | 22.8 ± 3.5 | 9 |
| Peltonen, JE 2012 [88] | male | adults | cycle ergometry | 7.7 ± 0.7 | 34.7 ± 4.4 | 10 |
| Peltoniemi, P 2001 [89] | male | adults | cycle ergometry | 7 ± 0.3 | 45.0 ± 2.0 | 12 |
| Poortmans, JR 1986 (a) [90] | male | adolescents | cycle ergometry | 7.3 ± 0.3 | 40.6 ± 1.3 | 9 |
| Poortmans, JR 1986 (b) | male | adolescents | cycle ergometry | 11.4 ± 0.9 | 38.5 ± 1.0 | 8 |
| Raguso, CA 1995 [91] | male | adults | cycle ergometry | 8 ± 0.7 | 40.5 ± 2.0 | 7 |
| Reddy, R 2019 [92] | both (m/w = 4/6) | adults | treadmill | 7.4 ± 1.0 | 46.8 ± 11.6 | 10 |
| Rigla, M 2000 (a) bl. [93] | both (m/w = 7/7) | adults | treadmill | 6.5 ± 0.8 | 33.7 ± 7.0 | 14 |
| Rigla, M 2000 (b) pi. | both (m/w = 7/7) | adults | treadmill | 6.7 ± 1.0 | 38.5 ± 7.7 | 14 |
| Rigla, M 2001 (a) bl. [94] | both (m/w = 7/7) | adults | other | 6.5 ± 0.8 | 33.7 ± 7.0 | 14 |
| Rigla, M 2001 (b) pi. | both (m/w = 7/7) | adults | other | 6.7 ± 1.0 | 38.5 ± 7.7 | 14 |
| Rissanen, APE 2015 [95] | male | adults | cycle ergometry | 7.4 ± 0.9 | 40.0 ± 3.0 | 7 |
| Rissanen, APE 2018 (a) bl. [96] | male | adults | cycle ergometry | 7.3 ± 0.9 | 38.0 ± 4.0 | 8 |
| Rissanen, APE 2018 (b) pi. | male | adults | cycle ergometry | 7.5 ± 1.1 | 41.0 ± 3.0 | 8 |
| Roberts, TJ 2018 (1) [97] | both (m/w = 29/11) | adults | cycle ergometry | 7.7 ± 1.3 | 32.0 ± 10.0 | 40 |
| Roberts, TJ 2018 (2) [98] | both (m/w = 13/7) | adults | cycle ergometry | 8.1 ± 3.9 | 38.0 ± 9.0 | 20 |
| Roberts, TJ 2020 [99] | both (m/w = 24/10) | adults | cycle ergometry | 7.8 ± 1.3 | 33.0 ± 10.0 | 34 |
| Robitaille, M 2007 [100] | both (m/w = 5/3) | adults | cycle ergometer | 7.4 ± 0.4 | 42.9 ± 10.3 | 8 |
| Roche, DM 2008 (a) [101] | male | adolescents | treadmill | 9.4 ± 1.0 | 43.2 ± 7.3 | 15 |
| Roche, DM 2008 (b) | female | adolescents | treadmill | 9.8 ± 1.7 | 39.2 ± 9.0 | 14 |
| Roche, DM 2008 (c) | both | adolescents | treadmill | 9.6 ± 1.4 | 41.4 ± 8.2 | 29 |
| Rowland, TW 1992 [102] | male | adolescents | cycle ergometry | 11.3 ± 3.0 | 51.5 ± 5.8 | 11 |
| Roy-Fleming, A 2019 [103] | both (m/w = 11/11) | adults | cycle ergometry | 7.3 ± 1.0 | 32.6 ± 7.1 | 22 |
| Sandoval, DA 2004 [104] | both (m/w = 14/13) | adults | cycle ergometry | 7.8 ± 0.2 | 28.0 ± 2.0 | 27 |
| Schneider, SH 1992 (a) [105] | both (m/w = 12/4) | adults | cycle ergometry | 12.6 ± 0.8 | 26.6 ± 1.5 | 16 |
| Schneider, SH 1992 (b) | both (m/w = 25/14) | adults | cycle ergometry | 11.5 ± 0.6 | 35.4 ± 1.9 | 39 |
| Seeger, JPH 2011 [106] | both (m/w = 4/5) | children | treadmill | 7.9 ± 0.6 | 44.0 ± 5.9 | 9 |
| Shetty, VB 2018 [107] | both (m/w = 4/4) | adults | cycle ergometry | 8.0 ± 0.7 | 34.5 ± 10.9 | 8 |
| Singhvi, A 2014 [108] | both (m/w = 47%/53%) | adolescents | treadmill | 8.42 ± 0.9 | 46.6 ± 6.8 | 20 |
| Stettler, C 2005 [109] | male | adults | cycle ergometry | 7.4 ± 0.7 | 44.9 ± 8.0 | 8 |
| Stewart, CJ 2017 [110] | male | adults | other | 7.4 ± 0.4 | 51.3 ± 2.2 | 10 |
| Tagougui, S (1a) 2015 [111] | male | adults | cycle ergometry | 6.6 ± 0.7 | 40.9 ± 9.3 | 11 |
| Tagougui, S (1b) 2015 | both (m/w = 7/5) | adults | cycle ergometry | 9.1 ± 0.7 | 34.6 ± 7.2 | 12 |
| Tagougui, S (2a) 2015 [112] | both (m/w = 7/1) | adults | other | 6.8 ± 0.7 | 39.6 ± 8.5 | 8 |
| Tagougui, S (2b) 2015 | both (m/w = 6/4) | adults | other | 9.0 ± 0.7 | 34.6 ± 7.1 | 10 |
| Tagougui, S 2020 [113] | both (m/w = 20/10) | adolescents/adults | treadmill | 7.6 ± 1.0 | 38.9 ± 10.7 | 30 |
| Tonoli, C 2015 [114] | both (m/w = 8/2) | adults | cycle ergometry | 7.0 ± 0.2 | 52.5 ± 2.7 | 10 |
| Trigona, B 2010 [115] | both (m/w = 17/15) | adolescents | treadmill | 8.2 ± 0.2 | 45.5 ± 1.44 | 32 |
| Tuominen, JA 1997 [116] | both (m/w = 6/1) | adults | cycle ergometry | 7.7 ± 0.3 | 46.0 ± 1.0 | 7 |
| Turinese, I 2017 [117] | both (m/w = 13/4) | adults | cycle ergometry | 7.4 ± 0.1 | 28.1 ± 1.3 | 17 |
| Tuttle, KR 1988 [118] | both (m/w = 8/5) | adults | cycle ergometry | 8.8 ± 1.6 | 36.4 ± 5.9 | 13 |
| Valletta, JJ 2014 [119] | both (m/w = 11/12) | adults | treadmill | 7.7 ± 1.3 | 39.9 ± 8.4 | 23 |
| Veves, A 1997 (a) [120] | both (m/w = 20/3) | adults | treadmill | 8.3 ± 1.4 | 54.0 ± 8.1 | 23 |
| Veves, A 1997 (b) | both (m/w = 4/3) | adults | treadmill | 9.8 ± 1.2 | 42.2 ± 11.6 | 7 |
| Veves, A 1997 (c) | both (m/w = 11/7) | adults | treadmill | 8.9 ± 1.5 | 36.7 ± 9.6 | 5 |
| Waclawovsky, G 2016 [121] | male | adults | cycle ergometry | 7.7 ± 0.2 | 37.1 ± 1.4 | 14 |
| Wallberg-Henriksson, H 1982 (a) bl. [122] | male | adults | cycle ergometry | 10.4 ± 0.7 | 42.1 ± 2.1 | 9 |
| Wallberg-Henriksson, H 1982 (b) pi. | male | adults | cycle ergometry | 11.3 ± 0.5 | 45.3 ± 2.2 | 9 |
| Wallberg-Henriksson, H 1986 (a) bl. [123] | female | adults | cycle ergometry | 10.4 ± 0.6 | 30.2 ± 2.1 | 6 |
| Wallberg-Henriksson, H 1986 (b) pi. | female | adults | cycle ergometry | 10.4 ± 0.6 | 32.7 ± 2.1 | 6 |
| Wallberg-Henriksson, H 1986 (c) bl. | female | adults | cycle ergometry | 10.6 ± 0.6 | 28.0 ± 0.8 | 7 |
| Wallberg-Henriksson, H 1986 (d) pi. | female | adults | cycle ergometry | 10.6 ± 0.6 | 28.0 ± 0.8 | 7 |
| Wanke, T 1992 [124] | both (m/w = 31/5) | adults | cycle ergometry | 9.2 ± 2.7 | 33.7 ± 0.7 | 36 |
| West, DJ 2011 (a) [27] | both (m/w = 7/1) | adults | treadmill | 8 ± 0.2 | 35.8 ± 0.6 | 8 |
| West, DJ 2011 (b) | both (m/w = 7/1) | adults | treadmill | 8 ± 0.2 | 34.6 ± 0.5 | 8 |
| Wilson, LC 2017 [125] | both (m/w = 12/11) | adults | cycle ergometry | 8.4 ± 3.7 | 32.0 ± 9.0 | 23 |
| Yardley, JE 2012 [126] | both (m/w = 10/2) | adolescents/adults | treadmill | 7.1 ± 1.1 | 51.2 ± 10.8 | 12 |
| Yardley, JE 2013 (1) [127] | both (m/w = 10/2) | adults | treadmill | 7.1 ± 1.1 | 51.2 ± 10.8 | 12 |
| Yardley, JE 2013 (2) [128] | both (m/w = 10/2) | adults | other | 7.1 ± 1.1 | 51.2 ± 10.8 | 12 |
| Yardley, JE 2013 (3a) [129] | both | adolescents/adults | cycle ergometry/running | 7.2 ± 1.2 | 46.4 ± 10.1 | 9 |
| Yardley, JE 2013 (3b) | both | adolescents/adults | cycle ergometry/running | 7.3 ± 1.1 | 48.6 ± 7.8 | 10 |
| Zaharieva, DP 2019 [130] | both (m/w = 4/13) | adults | treadmill | 6.5 ± 0.5 | 41.6 ± 5.9 | 17 |
| Zaharieva, DP 2016 [131] | both (m/w = 5/8) | adults | treadmill | 7.4 ± 0.8 | 46.6 ± 12.7 | 13 |
| Zaharieva, DP 2017 [132] | both (m/w = 6/6) | adults | treadmill | 7 ± 0.9 | 50.1 ± 13.7 | 12 |
| Zebrowska, A 2018 (a) [133] | undefined | adults | cycle ergometry | 7.2 ± 0.41 | 43.9 ± 7.8 | 14 |
| Zebrowska, A 2018 (b) | undefined | adults | cycle ergometry | 7.2 ± 0.41 | 40.3 ± 7.3 | 14 |
Types of exercise identified as “other” were conducted outside on a track with a spirometric wireless device or if the type of exercise during CPX was not specified.
3.1. Primary Endpoint
The overall pooled mean values were 8.1% [95% CI: 7.9–8.3%] for HbA1c and 38.5 (37.3–39.6) mL/kg/min for VO2max. Studies including participants with a mean HbA1c ≥7.5% had a significantly lower VO2max with 36.9 [35.9–38.1] mL/kg/min in comparison to those having an HbA1c < 7.5 with 41.3 (39.2–43.4) mL/kg/min (p < 0.001). For both, VO2max (I2 = 98.09%) and HbA1c (I2 = 96.77%) high heterogeneity for study results was observed.
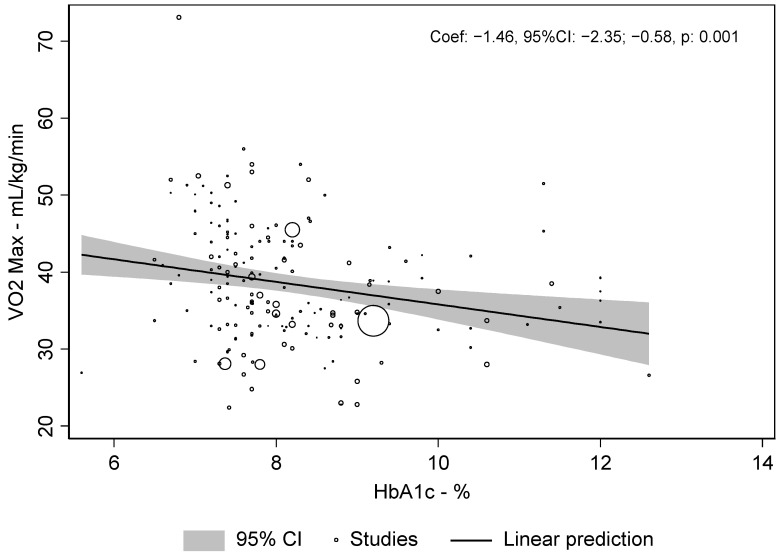
There was a weak, but significant correlation between VO2max and HbA1c of r = −0.19 (p < 0.001). The meta-regression revealed a slope between VO2max and HbA1c of −1.46 [−2.35–−0.58], p < 0.001), i.e., for each increase in HbA1c of 1% the relative VO2max was lower by 1.46 mL/kg/min. This association is shown in Figure 3.
Figure 3.
Meta-regression dot plot for HbA1c and VO2max.
3.2. Subgroup Analysis
Subgroup analyses were conducted for sex, age group and type of exercise. The highest pooled mean VO2max was observed in studies including only male participants (44.3 [41.9–46.6] mL/kg/min vs. 32.2 (28.5–35.8) mL/kg/min in women and 36.6 (35.4–37.7) mL/kg/min in studies including both sexes, p < 0.001). Overall participants had a mean pooled HbA1c of 8.1% (95% CI: 7.9–8.3%) with male participants at 7.8% (7.5–8.1) and female participants at 8.8% (8.2–9.5) (p = 0.01).
Only minor differences were observed for VO2max across the age groups: adolescents had a VO2max of 37.6 (35.4–39.7) mL/kg/min, adolescents/adults 38.9 (35.9–41.9) mL/kg/min, adults 38.6 (37.0–40.1) mL/kg/min and non-specified groups 38.4 (33.8–42.9) (p = 0.857). Only two studies reported on VO2max and HbA1c in children, those results are included in Supplemental Table S5 under the subgroup ‘other’. In contrast, clear differences were seen for VO2max for different types of exercise; cycling led to a mean pooled VO2max of 36.9 (35.5–38.2) mL/kg/min vs. 43.5 (41.4–45.5) mL/kg/min with treadmill and 40.4 (37.5–43.2) mL/kg/min for other types of exercise to (p < 0.001).
3.3. Multivariate Meta-Regression
Multivariate meta-regressions for VO2max in association with HbA1c, sex, age group and type of exercise were conducted. The results are shown in Table 2. Univariate Meta-regression can be found in the supplementary material.
Table 2.
Multivariate meta-regression of study outcomes.
| Coefficient [95% CI] | p Value | |
|---|---|---|
| HbA1c (continuous) | −0.78 [−1.56–−0.003] | 0.049 |
| Gender | ||
| Both | Reference | |
| Female | −2.42 [−5.93–1.09] | 0.176 |
| Male | 9.21 [7.03–11.4] | <0.001 |
| Age | ||
| Other | Reference | |
| Adolescents | −0.51 [−5.36–4.33] | 0.836 |
| Adolescents/Adults | 0.75 [−4.43–5.93] | 0.777 |
| Adults | −2.46 [−7.15–2.23] | 0.304 |
| Exercise type | ||
| Other | Reference | |
| Cycle ergometer | −3.84 [−7.18–−0.5] | 0.024 |
| Treadmill | 4.24 [0.48–8.0] | 0.027 |
4. Discussion
Our systematic review and meta-analysis showed that HbA1c and VO2max are inversely, albeit weakly associated. Furthermore, if groups were separated for HbA1c, significant differences in VO2max between low (<7.5%) and high (≥7.5%) values were found. Additional subgroup analyses showed that VO2max was highest in male (in comparison to female) participants whereas age only had a small, insignificant effect on VO2max and on the impact of HbA1c on VO2max.
Physical exercise has previously shown to not necessarily decrease HbA1c for a variety of reasons [134,135]. Individuals with T1DM are advised to reduce insulin doses in preparation for specific exercise sessions, which may elevate BG and eventually (at least with regular physical exercise) HbA1c [136]. In addition, patients are advised to supplement CHO with falling glucose values during exercise [136]. Even though the amounts of administered CHO are small, they may weaken the positive exercise induced effects on body mass and glycemic control.
Moreover, previous exercise studies have shown that slightly elevated BG levels or CHO supplementation during physical exercise may increase the performance of individuals without T1DM [136]. In combination with elevated pre-exercise BG values (due to insulin reductions) and higher post-exercise BG levels (in response to supplemented CHO during exercise), HbA1c might be negatively impacted in those people with T1DM who are physically active, and fear of hypoglycemia might be another contributor.
However, a large multi-center study demonstrated that physical activity has a positive impact on glycemic control but also on diabetes-related comorbidities [137]. This is supported by the study from King et al. that found a positive association between glycemic control and the amount of physical activity in children with T1DM [138]. These studies are reflective of the results from our systematic review and meta-analysis that show an inverse relationship between HbA1c and VO2max, highlighting that a 1% increase in HbA1c decreases VO2max by 1.46 mL/kg/min. This may appear negligible at first, but Laukkanen et al. have shown that a decrease of 1 mL/kg/min of VO2max is associated with a 9% increase in all-cause mortality in a population-based study of 579 men without diabetes [139]. VO2max has previously been suggested to predict longevity. While still a matter of research, an elevated VO2max allows individuals to be more active which may increase well-being and longevity [140]. It should be noted that HbA1c worsens between the ages of 8–18, while from the age of 16 it steadily improves due to a higher awareness of diabetes management. In addition, puberty and hormonal changes at that time contribute to a more complicated glycemic management that may ease over time [141]. Adolescents in our study were already in the ‘steadily improving’ age range hence the impact of puberty and hormonal changes are not as pronounced or influential on our study results. Furthermore, adolescents with T1DM do not necessarily have a smaller VO2max compared to adults since studies in healthy individuals [142] and individuals with T1DM [143] align with our overall findings regarding VO2max.
Even though the association was weak in our results, HbA1c has shown to reduce skeletal muscle mitochondrial ATP production, which consequently reduces performance, deteriorating VO2max [144]. In individuals with an increased HbA1c, capillary density around skeletal musculature has also been shown to be decreased [145]. This may in addition lead to compromised oxygen supply systems influencing an individual’s functional capacity.
These studies and our systematic review and meta-analysis are not without limitations. Although still considered the gold standard of glycemic control, HbA1c may not be the ideal parameter to detect the effects of physical exercise on glucose control in individuals with T1DM as it is an average of low and high BG values over a rather long period of time. Low BG values will reduce HbA1c although they are not exactly an indicator of good glycemic control [146]. On the other hand, exercise-induced hyperglycemic BG values which are often accepted during physical activity will increase HbA1c which might cast doubt on the benefit of exercise in people with T1DM. Another contributor to the effects seen in our analysis could be diabetes duration. This may have an impact on HbA1c but also on VO2max since it may decrease over time with insufficient training. However, it might not necessarily have an impact since diabetes duration could be influential once individuals with T1DM have gained more knowledge about their own diabetes which could thus improve their overall glycemic control and engagement in physical activities increasing VO2max. Unfortunately, this could not be included in our systematic review and meta-analysis, since the majority of the studies did not show the diabetes duration in detail, omitting this important detail which should be considered in future studies.
Future studies should focus on more differentiated parameters than HbA1c such as time in range which represents the standard parameter to assess quality of glycemic control in users of continuous glucose monitoring systems. Hypoglycemic and hyperglycemic glucose values do not compensate for each other in time in range. Therefore, time in range might be a more appropriate parameter to evaluate quality of glycemic control in particular for interventions potentially triggering hypoglycemia, including physical activity. It must be mentioned, that several confounding parameters may have an impact on HbA1c without influencing VO2max. This could be an adaption in dietary behavior, e.g., meal timing or overall carbohydrate intake, or this could be a change in therapy from pen to pump therapy or an upgrade to a novel insulin analogue that may lower HbA1c immediately. Future studies on this important research topic will hopefully investigate additional parameters potentially affecting HbA1c and its association with VO2max, such as body mass, comorbidities and socioeconomic status—parameters that could not be included in this meta-analysis since as they were either not measured or reported insufficiently, leaving important information about the association of HbA1c and VO2max unexplored.
In our systematic review and meta-analysis, we chose the gold standard parameters for glycemic control and maximum oxygen uptake, HbA1c and VO2max, since they are the most popular and most often applied parameters in diabetes research, but VO2max has its limitations, too. It is unable to fully represent the entire spectrum of physical performance since cardiological (heart rate max), metabolic (lactate max) and physiological parameters such as power and speed are not reflected. Since we solely included studies with individuals that conducted exercise tests until volitional exhaustion for the determination of VO2max, results from our study cannot be transferred to all types of physical exercise.
Nevertheless, future studies are needed to investigate additional parameters that may impact long-term glycemic control and its association with VO2max in individuals with T1DM.
5. Conclusions
This meta-analysis demonstrates an inverse association between physical performance and HbA1c showing an increase in VO2max with decreasing HbA1c. Further studies relating to time in range will be needed to confirm a positive impact of glucose control on physical performance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo12111017/s1, Supplemental Material. Univariate Regression; Table S1: Pubmed search, July 2020; Table S2: Embase search, July 2020; Table S3: Web of science search, July 2020; Table S4: Cochrane search, July 2020; Table S5: Statistical analysis of HbA1c of all included groups; Figure S1: Funnel Plot title.
Author Contributions
M.L.E. wrote the first draft of the manuscript, conceptualized and designed the literature search, conducted the literature search, performed data curation, visualization, supervision, statistical analyses and wrote the manuscript. F.J.R.D. supported with the literature search with statistical analyses and reviewed and edited the manuscript. F.A. (Faisal Aziz) performed the statistical and formal analyses. F.A. (Felix Aberer), F.J.R.D., F.A. (Faisal Aziz), T.H., H.S. and O.M. supported with writing the manuscript and reviewing and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest pertinent to the systematic review and meta-analysis.
Funding Statement
The APC were funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—491183248 and the Open Access Publishing Fund of the University of Bayreuth.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Diabetes Association Glycemic targets: Standards of medical care in diabetes. Diabetes Care. 2019;42:S61–S70. doi: 10.2337/dc19-S006. [DOI] [PubMed] [Google Scholar]
- 2.Colberg S.R., Sigal R.J., Yardley J.E., Riddell M.C., Dunstan D.W., Dempsey P.C., Horton E.S., Castorino K., Tate D.F. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tikkanen-Dolenc H., Wadén J., Forsblom C., Harjutsalo V., Thorn L.M., Saraheimo M., Elonen N., Hietala K., Summanen P., Heikki G., et al. Frequent physical activity is associated with reduced risk of severe diabetic retinopathy in type 1 diabetes on behalf of the FinnDiane Study Group. Acta Diabetol. 2020;57:527–534. doi: 10.1007/s00592-019-01454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon T.P.J., Malin S.K., Karstoft K., Knudsen S.H., Haus J.M., Laye M.J., Kirwan J.P. Association Between Cardiorespiratory Fitness and the Determinants of Glycemic Control Across the Entire Glucose Tolerance Continuum. Diabetes Care. 2015;35:921–929. doi: 10.2337/dc14-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narendran P., Solomon T.P., Kennedy A., Chimen M., Andrews R.C. The time has come to test the beta cell preserving effects of exercise in patients with new onset type 1 diabetes. Diabetologia. 2015;58:10–18. doi: 10.1007/s00125-014-3412-8. [DOI] [PubMed] [Google Scholar]
- 6.Eckstein M.L., Farinha J.B., McCarthy O., West D.J., Yardley J.E., Bally L., Zueger T., Stettler C., Boff W., Reischak-Oliveira A., et al. Differences in Physiological Responses to Cardiopulmonary Exercise Testing in Adults With and Without Type 1 Diabetes: A Pooled Analysis. Diabetes Care. 2020:dc201496. doi: 10.2337/dc20-1496. [DOI] [PubMed] [Google Scholar]
- 7.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br. Med. J. 2011;343:889–893. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 10.American College of Sports Medicine . ACSM’s Resources for Clinical Exercise Physiology. 7th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2009. [Google Scholar]
- 11.Dalleck L.C., Tischendorf J.S. Encyclopedia of Lifestyle Medicine & Health. Sage; Thousand Oaks, CA, USA: 2012. Guidelines for Exercise Testing and Prescription (ACSM) p. 472. [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abraham M.B., Davey R.J., Cooper M.N., Paramalingam N., O’Grady M.J., Ly T.T., Jones T.W., Fournier P.A., Davis E.A. Reproducibility of the plasma glucose response to moderate-intensity exercise in adolescents with Type 1 diabetes. Diabet. Med. 2017;34:1291–1295. doi: 10.1111/dme.13395. [DOI] [PubMed] [Google Scholar]
- 14.Adolfsson P., Nilsson S., Albertsson-Wikland K., Lindblad B. Hormonal response during physical exercise of different intensities in adolescents with type 1 diabetes and healthy controls. Pediatr. Diabetes. 2012;13:587–596. doi: 10.1111/j.1399-5448.2012.00889.x. [DOI] [PubMed] [Google Scholar]
- 15.Al Khalifah R.A., Suppère C., Haidar A., Rabasa-Lhoret R., Ladouceur M., Legault L. Association of aerobic fitness level with exercise-induced hypoglycaemia in Type 1 diabetes. Diabet. Med. 2016;33:1686–1690. doi: 10.1111/dme.13070. [DOI] [PubMed] [Google Scholar]
- 16.Atalay M., Laaksonen D.E., Niskanen L., Uusitupa M., Hänninen O., Sen C.K. Altered antioxidant enzyme defences in insulin-dependent diabetic men with increased resting and exercise-induced oxidative stress. Acta Physiol. Scand. 1997;161:195–201. doi: 10.1046/j.1365-201X.1997.00200.x. [DOI] [PubMed] [Google Scholar]
- 17.Austin A., Warty V., Arslanian S. The Relationship of Physical Fitness to Lipid and Lipoprotein(a) Levels in Adolescents With IDDM. Diabetes Care. 1993;16:421–425. doi: 10.2337/diacare.16.2.421. [DOI] [PubMed] [Google Scholar]
- 18.Bak J.F., Jacobsen U.K., Jørgensen F.S., Pedersen O. Insulin receptor function and glycogen synthase activity in skeletal muscle biopsies from patients with insulin-dependent diabetes mellitus: Effects of physical training. J. Clin. Endocrinol. Metab. 1989;69:158–164. doi: 10.1210/jcem-69-1-158. [DOI] [PubMed] [Google Scholar]
- 19.Baldi J.C., Cassuto N.A., Foxx-Lupo W.T., Wheatley C.M., Snyder E.M. Glycemic status affects cardiopulmonary exercise response in athletes with type I diabetes. Med. Sci. Sports Exerc. 2010;42:1454–1459. doi: 10.1249/MSS.0b013e3181d1fdb3. [DOI] [PubMed] [Google Scholar]
- 20.Bally L., Zueger T., Buehler T., Dokumaci A.S., Speck C., Pasi N., Ciller C., Paganini D., Feller K., Loher H., et al. Metabolic and hormonal response to intermittent high-intensity and continuous moderate intensity exercise in individuals with type 1 diabetes: A randomised crossover study. Diabetologia. 2016;59:776–784. doi: 10.1007/s00125-015-3854-7. [DOI] [PubMed] [Google Scholar]
- 21.Bally L., Zueger T., Pasi N., Carlos C., Paganini D., Stettler C. Accuracy of continuous glucose monitoring during differing exercise conditions. Diabetes Res. Clin. Pract. 2016;112:1–5. doi: 10.1016/j.diabres.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Baraldi E., Monciotti C., Filippone M., Santuz P., Magagnin G., Zanconato S., Zacchello F. Gas exchange during exercise in diabetic children. Pediatr. Pulmonol. 1992;13:155–160. doi: 10.1002/ppul.1950130306. [DOI] [PubMed] [Google Scholar]
- 23.Benbassat C.A., Stern E., Kramer M., Lebzelter J., Blum I., Fink G. Pulmonary function in patients with diabetes mellitus. Am. J. Med. Sci. 2001;322:127–132. doi: 10.1097/00000441-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Bjornstad P., Cree-Green M., Baumgartner A., Coe G., Reyes Y.G., Schäfer M., Pyle L., Regensteiner J.G., Reusch J.E.B., Nadeau K.J. Achieving ADA/ISPAD clinical guideline goals is associated with higher insulin sensitivity and cardiopulmonary fitness in adolescents with type 1 diabetes: Results from RESistance to InSulin in Type 1 ANd Type 2 diabetes (RESISTANT) and Effects of MEtform. Pediatr. Diabetes. 2018;19:436–442. doi: 10.1111/pedi.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjornstad P., Cree-Green M., Baumgartner A., Maahs D.M., Cherney D.Z., Pyle L., Regensteiner J.G., Reusch J.E., Nadeau K.J. Renal function is associated with peak exercise capacity in adolescents with type 1 diabetes. Diabetes Care. 2015;38:126–131. doi: 10.2337/dc14-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boff W., Da Silva A.M., Farinha J.B., Rodrigues-Krause J., Reischak-Oliveira A., Tschiedel B., Puñales M., Bertoluci M.C. Superior effects of high-intensity interval vs. moderate-intensity continuous training on endothelial function and cardiorespiratory fitness in patients with type 1 diabetes: A randomized controlled trial. Front. Physiol. 2019;10:1–10. doi: 10.3389/fphys.2019.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bracken R.M., Page R., Gray B., Kilduff L.P., West D.J., Stephens J.W., Bain S.C. Isomaltulose improves glycemia and maintains run performance in type 1 diabetes. Med. Sci. Sports Exerc. 2012;44:800–808. doi: 10.1249/MSS.0b013e31823f6557. [DOI] [PubMed] [Google Scholar]
- 28.Bracken R.M., West D.J., Stephens J.W., Kilduff L.P., Luzio S., Bain S.C. Impact of pre-exercise rapid-acting insulin reductions on ketogenesis following running in Type 1 diabetes. Diabet. Med. 2011;28:218–222. doi: 10.1111/j.1464-5491.2010.03162.x. [DOI] [PubMed] [Google Scholar]
- 29.Brazeau A.S., Leroux C., Mircescu H., Rabasa-Lhoret R. Physical activity level and body composition among adults with Type1 diabetes. Diabet. Med. 2012;29:402–408. doi: 10.1111/j.1464-5491.2012.03757.x. [DOI] [PubMed] [Google Scholar]
- 30.Brazeau A.S., Mircescu H., Desjardins K., Dubé M.C., Weisnagel S.J., Lavoie C., Rabasa-Lhoret R. The Barriers to Physical Activity in Type 1 Diabetes (BAPAD-1) scale: Predictive validity and reliability. Diabetes Metab. 2012;38:164–170. doi: 10.1016/j.diabet.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Brugnara L., Vinaixa M., Murillo S., Samino S., Rodriguez M.A., Beltran A., Lerin C., Davison G., Correig X., Novials A. Metabolomics approach for analyzing the effects of exercise in subjects with type 1 diabetes mellitus. PLoS ONE. 2012;7:2–9. doi: 10.1371/journal.pone.0040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bussau V.A., Ferreira L.D., Jones T.W., Fournier P.A. The 10-s Maximal Sprint. Diabetes Care. 2006;29:601–606. doi: 10.2337/diacare.29.03.06.dc05-1764. [DOI] [PubMed] [Google Scholar]
- 33.Bussau V.A., Ferreira L.D., Jones T.W., Fournier P.A. A 10-s sprint performed prior to moderate-intensity exercise prevents early post-exercise fall in glycaemia in individuals with type 1 diabetes. Diabetologia. 2007;50:1815–1818. doi: 10.1007/s00125-007-0727-8. [DOI] [PubMed] [Google Scholar]
- 34.Campaigne B.N., Wallberg-henriksson H., Gunnarsson R. Glucose and Insulin Responses in Relation to Insulin Dose and Caloric Intake 12 h After Acute Physical Exercise in Men With IDDM. Diabetes Care. 1987;10:716–721. doi: 10.2337/diacare.10.6.716. [DOI] [PubMed] [Google Scholar]
- 35.Campbell M.D., Walker M., Trenell M.I., Jakovljevic D.G., Stevenson E.J., Bracken R.M., Bain S.C., West D.J. Large pre-and postexercise rapid-acting insulin reductions preserve glycemia and prevent early- but not late-onset hypoglycemia in patients with type 1 diabetes. Diabetes Care. 2013;36:2217–2224. doi: 10.2337/dc12-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell M.D., Walker M., Trenell M.I., Luzio S., Dunseath G., Tuner D., Bracken R.M., Bain S.C., Russell M., Stevenson E.J., et al. Metabolic implications when employing heavy pre- and post-exercise rapid-acting insulin reductions to prevent hypoglycaemia in type 1 diabetes patients: A randomised clinical trial. PLoS ONE. 2014;9:e97143. doi: 10.1371/journal.pone.0097143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell M.D., Walker M., Trenell M.I., Stevenson E.J., Turner D., Bracken R.M., Shaw J.A., West D.J. A low-glycemic index meal and bedtime snack prevents postprandial hyperglycemia and associated rises in inflammatory markers, providing protection from early but not late nocturnal hypoglycemia following evening exercise in type 1 diabetes. Diabetes Care. 2014;37:1845–1853. doi: 10.2337/dc14-0186. [DOI] [PubMed] [Google Scholar]
- 38.Campbell M.D., West D.J., Bain S.C., Kingsley M.I.C., Foley P., Kilduff L., Turner D., Gray B., Stephens J.W., Bracken R.M. Simulated games activity vs continuous running exercise: A novel comparison of the glycemic and metabolic responses in T1DM patients. Scand. J. Med. Sci. Sport. 2015;25:216–222. doi: 10.1111/sms.12192. [DOI] [PubMed] [Google Scholar]
- 39.Campbell M.D., Walker M., Bracken R.M., Turner D., Stevenson E.J., Gonzalez J.T., Shaw J.A., West D.J. Insulin therapy and dietary adjustments to normalize glycemia and prevent nocturnal hypoglycemia after evening exercise in type 1 diabetes: A randomized controlled trial. BMJ Open Diabetes Res. Care. 2015;3:1–8. doi: 10.1136/bmjdrc-2015-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chokkalingam K., Tsintzas K., Norton L., Jewell K., Macdonald I.A., Mansell P.I. Exercise under hyperinsulinaemic conditions increases whole-body glucose disposal without affecting muscle glycogen utilisation in type 1 diabetes. Diabetologia. 2007;50:414–421. doi: 10.1007/s00125-006-0520-0. [DOI] [PubMed] [Google Scholar]
- 41.de Jesus Í.C., Mascarenhas L.P.G., de Lima V.A., Decimo J.P., Nesi-França S., Leite N. Maximal fat oxidation during aerobic exercise in adolescents with type 1 diabetes. Rev. Bras. Med. do Esporte. 2019;25:299–304. doi: 10.1590/1517-869220192504189259. [DOI] [Google Scholar]
- 42.De Lima V.A., Mascarenhas L.P.G., Decimo J.P., De Souza W.C., Monteiro A.L.S., Lahart I., França S.N., Leite N. Physical activity levels of adolescents with type 1 diabetes physical activity in T1D. Pediatr. Exerc. Sci. 2017;29:213–219. doi: 10.1123/pes.2016-0199. [DOI] [PubMed] [Google Scholar]
- 43.D’hooge R., Hellinckx T., Van Laethem C., Stegen S., De Schepper J., Van Aken S., Dewolf D., Calders P. Influence of combined aerobic and resistance training on metabolic control, cardiovascular fitness and quality of life in adolescents with type 1 diabetes: A randomized controlled trial. Clin. Rehabil. 2011;25:349–359. doi: 10.1177/0269215510386254. [DOI] [PubMed] [Google Scholar]
- 44.Dovc K., Macedoni M., Bratina N., Lepej D., Nimri R., Atlas E., Muller I., Kordonouri O., Biester T., Danne T., et al. Closed-loop glucose control in young people with type 1 diabetes during and after unannounced physical activity: A randomised controlled crossover trial. Diabetologia. 2017;60:2157–2167. doi: 10.1007/s00125-017-4395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ebeling P., Tuominen J.A., Bourey R., Koranyi L., Koivisto V.A. Athletes with IDDM exhibit impaired metabolic control and increased lipid utilization with no increase in insulin sensitivity. Diabetes. 1995;44:471–477. doi: 10.2337/diab.44.4.471. [DOI] [PubMed] [Google Scholar]
- 46.Farinha J.B., Ramis T.R., Vieira A.F., Macedo R.C.O., Rodrigues-Krause J., Boeno F.P., Schroeder H.T., Müller C.H., Boff W., Krause M., et al. Glycemic, inflammatory and oxidative stress responses to different high-intensity training protocols in type 1 diabetes: A randomized clinical trial. J. Diabetes Complicat. 2018;32:1124–1132. doi: 10.1016/j.jdiacomp.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Faulkner M.S., Michaliszyn S.F., Hepworth J.T. A personalized approach to exercise promotion in adolescents with type 1 diabetes. Pediatr. Diabetes. 2010;11:166–174. doi: 10.1111/j.1399-5448.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faulkner M.S., Quinn L., Rimmer J.H., Rich B.H. Cardiovascular endurance and heart rate variability in adolescents with type 1 or type 2 diabetes. Biol. Res. Nurs. 2005;7:16–29. doi: 10.1177/1099800405275202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fintini D., Di Giacinto B., Brufani C., Cafiero G., Patera P.I., Turchetta A., Giordano U., Nobili V., Pelliccia A., Calzolari A., et al. Impaired energy expenditure despite normal cardiovascular capacity in children with type 1 diabetes. Horm. Res. Paediatr. 2012;78:1–7. doi: 10.1159/000339465. [DOI] [PubMed] [Google Scholar]
- 50.Franc S., Daoudi A., Pochat A., Petit M.H., Randazzo C., Petit C., Duclos M., Penfornis A., Pussard E., Not D., et al. Insulin-based strategies to prevent hypoglycaemia during and after exercise in adult patients with type 1 diabetes on pump therapy: The DIABRASPORT randomized study. Diabetes, Obes. Metab. 2015;17:1150–1157. doi: 10.1111/dom.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Francis S.L., Singhvi A., Tsalikian E., Tansey M.J., Janz K.F. Cross-validation of single-stage treadmill tests for predicting aerobic fitness in adolescents with type I diabetes. Pediatr. Exerc. Sci. 2015;27:396–403. doi: 10.1123/pes.2014-0146. [DOI] [PubMed] [Google Scholar]
- 52.Fuchsjäger-Mayrl G., Pleiner J., Wiesinger G.F., Sieder A.E., Quittan M., Nuhr M.J., Francesconi C., Seit H.P., Francesconi M., Schmetterer L., et al. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care. 2002;25:1795–1801. doi: 10.2337/diacare.25.10.1795. [DOI] [PubMed] [Google Scholar]
- 53.Giani E., Macedoni M., Barilli A., Petitti A., Mameli C., Bosetti A., Cristiano A., Radovanovic D., Santus P., Zuccotti G.V. Performance of the Flash Glucose Monitoring System during exercise in youth with Type 1 diabetes. Diabetes Res. Clin. Pract. 2018;146:321–329. doi: 10.1016/j.diabres.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Goulding R.P., Roche D.M., Scott S.N., Koga S., Weston P.J., Marwood S. Limitations to exercise tolerance in type 1 diabetes: The role of pulmonary oxygen uptake kinetics and priming exercise. J. Appl. Physiol. 2020;128:1299–1309. doi: 10.1152/japplphysiol.00892.2019. [DOI] [PubMed] [Google Scholar]
- 55.Gray B.J., Page R., Turner D., West D.J., Campbell M.D., Bracken R.M. Improved end-stage high intensity performance but similar glycaemic responses after waxy barley starch ingestion compared to dextrose in type 1 diabetes. J. Sports Med. Phys. Fit. 2015;56:1392–1400. [PubMed] [Google Scholar]
- 56.Kj G., Tw J., Pa F. The decline in blood glucose levels is less with intermittent high-intensity compared with moderate exercise in individuals with type 1 diabetes. Diabetes Care. 2005;28:1289–1294. doi: 10.2337/diacare.28.6.1289. [DOI] [PubMed] [Google Scholar]
- 57.Guelfi K.J., Ratnam N., Smythe G.A., Jones T.W., Fournier P.A. Effect of intermittent high-intensity compared with continuous moderate exercise on glucose production and utilization in individuals with type 1 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007;292:E865–E870. doi: 10.1152/ajpendo.00533.2006. [DOI] [PubMed] [Google Scholar]
- 58.Gusso S., Hofman P., Lalande S., Cutfield W., Robinson E., Baldi J.C. Impaired stroke volume and aerobic capacity in female adolescents with type 1 and type 2 diabetes mellitus. Diabetologia. 2008;51:1317–1320. doi: 10.1007/s00125-008-1012-1. [DOI] [PubMed] [Google Scholar]
- 59.Gusso S., Pinto T.E., Baldi J.C., Robinson E., Cutfield W.S., Hofman P.L. Diastolic function is reduced in adolescents with type 1 diabetes in response to exercise. Diabetes Care. 2012;35:2089–2094. doi: 10.2337/dc11-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hägglund H., Uusitalo A., Peltonen J.E., Koponen A.S., Aho J., Tiinanen S., Seppänen T., Tulppo M., Tikkanen H.O. Cardiovascular autonomic nervous system function and aerobic capacity in type 1 diabetes. Front. Physiol. 2012;3:356. doi: 10.3389/fphys.2012.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heise T., Bain S.C., Bracken R.M., Zijlstra E., Nosek L., Stender-Petersen K., Rabøl R., Rowe E., Haahr H.L. Similar risk of exercise-related hypoglycaemia for insulin degludec to that for insulin glargine in patients with type 1 diabetes: A randomized cross-over trial. Diabetes Obes. Metab. 2016;18:196–199. doi: 10.1111/dom.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heyman E., Daussin F., Wieczorek V., Caiazzo R., Matran R., Berthon P., Aucouturier J., Berthoin S., Descatoire A., Leclair E., et al. Muscle oxygen supply and use in type 1 diabetes, from ambient air to the mitochondrial respiratory chain: Is there a limiting step? Diabetes Care. 2020;43:209–218. doi: 10.2337/dc19-1125. [DOI] [PubMed] [Google Scholar]
- 63.Heyman E., Delamarche P., Berthon P., Meeusen R., Briard D., Vincent S., DeKerdanet M., Delamarche A. Alteration in sympathoadrenergic activity at rest and during intense exercise despite normal aerobic fitness in late pubertal adolescent girls with type 1 diabetes. Diabetes Metab. 2007;33:422–429. doi: 10.1016/j.diabet.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Hilberg T., Eichler E., Gläser D., Schmidt V., Gabriel H.H.W. Platelet activity, reactivity and platelet-leukocyte conjugate formation before and after exhaustive or moderate exercise in patients with IDDM. Platelets. 2004;15:101–108. doi: 10.1080/09537100310001646941. [DOI] [PubMed] [Google Scholar]
- 65.Jenni S., Oetliker C., Allemann S., Ith M., Tappy L., Wuerth S., Egger A., Boesch C., Schneiter P., Diem P., et al. Fuel metabolism during exercise in euglycaemia and hyperglycaemia in patients with type 1 diabetes mellitus - A prospective single-blinded randomised crossover trial. Diabetologia. 2008;51:1457–1465. doi: 10.1007/s00125-008-1045-5. [DOI] [PubMed] [Google Scholar]
- 66.Jensen T., Richter E.A., Feldt-Rasmussen B., Kelbaek H., Deckert T. Impaired aerobic work capacity in insulin dependent diabetics with increased urinary albumin excretion. Br. Med. J. (Clin. Res. Ed). 1988;296:1352–1354. doi: 10.1136/bmj.296.6633.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Komatsu W.R., Neto T.L.B., Chacra A.R., Dib S.A. Aerobic exercise capacity and pulmonary function in athletes with and without type 1 diabetes. Diabetes Care. 2010;33:2555–2557. doi: 10.2337/dc10-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Komatsu W.R., Lima Gabbay M.A., Castro M.L., Saraiva G.L., Chacra A.R., Leite de Barros Neto T., Dib S.A. Aerobic exercise capacity in normal adolescents and those with type 1 diabetes mellitus. Pediatr. Diabetes. 2005;6:145–149. doi: 10.1111/j.1399-543X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- 69.Koponen A.S., Peltonen J.E., Päivinen M.K., Aho J.M., Hägglund H.J., Uusitalo A.L., Lindholm H.J., Tikkanen H.O. Low total haemoglobin mass, blood volume and aerobic capacity in men with type 1 diabetes. Eur. J. Appl. Physiol. 2013;113:1181–1188. doi: 10.1007/s00421-012-2532-4. [DOI] [PubMed] [Google Scholar]
- 70.Kornhauser C., Malacara J.M., Macías-Cervantes M.H., Rivera-Cisneros A.E. Effect of exercise intensity on albuminuria in adolescents with Type1 diabetes mellitus. Diabet. Med. 2012;29:70–73. doi: 10.1111/j.1464-5491.2011.03380.x. [DOI] [PubMed] [Google Scholar]
- 71.Laaksonen D.E., Atalay M., Niskanen L.K., Mustonen J., Sen C.K., Lakka T.A., Uusitupa M.I.J. Aerobic exercise and the lipid profile in type 1 diabetic men: A randomized controlled trial. Med. Sci. Sports Exerc. 2000;32:1541–1548. doi: 10.1097/00005768-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Laaksonen D.E., Atalay M., Niskanen L., Uusitupa M., Hänninen O., Sen C.K. Increased resting and exercise-induced oxidative stress in young IDDM men. Diabetes Care. 1996;19:569–574. doi: 10.2337/diacare.19.6.569. [DOI] [PubMed] [Google Scholar]
- 73.Landt K.W., Campaigne B.N., James F.W., Sperling M.A. Effects of exercise training on insulin sensitivity in adolescents with type I diabetes. Diabetes Care. 1985;8:461–465. doi: 10.2337/diacare.8.5.461. [DOI] [PubMed] [Google Scholar]
- 74.Lee M.J., Coast J.R., Hempleman S.C., Baldi J.C. Type 1 diabetes duration decreases pulmonary diffusing capacity during exercise. Respiration. 2016;91:164–170. doi: 10.1159/000443181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lehmann R., Kaplan V., Bingisser R., Bloch K.E., Spinas G.A. Impact of physical activity on cardiovascular risk factors in IDDM. Diabetes Care. 1997;20:1603–1611. doi: 10.2337/diacare.20.10.1603. [DOI] [PubMed] [Google Scholar]
- 76.Matthys D., Craen M., Wolf D.D.E., Walle J.V., Verhaaren H. Reduced decrease of peripheral vascular resistance during exercise in young type I diabetic patients. Diabetes Care. 1996;19:1286–1288. doi: 10.2337/diacare.19.11.1286. [DOI] [PubMed] [Google Scholar]
- 77.Mccarthy O., Eckstein M.L., Scott S.N., Fontana F.Y., Christiansen M.P., Stettler C., Fisher M., Bode B., Riddell M.C., Hayes C., et al. Glycemic responses to strenuous training in male professional cyclists with type 1 diabetes: A prospective observational study. BMJ Open Diabetes Res. Care. 2020:1–9. doi: 10.1136/bmjdrc-2020-001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McKewen M.W., Rehrer N.J., Cox C., Mann J. Glycaemic control, muscle glycogen and exercise performance in IDDM athletes on diets of varying carbohydrate content. Int. J. Sports Med. 1999;20:349–353. doi: 10.1055/s-2007-971143. [DOI] [PubMed] [Google Scholar]
- 79.Michaliszyn S.F., Shaibi G.Q., Quinn L., Fritschi C., Faulkner M.S. Physical fitness, dietary intake, and metabolic control in adolescents with type 1 diabetes. Pediatr. Diabetes. 2009;10:389–394. doi: 10.1111/j.1399-5448.2009.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moser O., Eckstein M.L., McCarthy O., Deere R., Bain S.C., Haahr H.L., Zijlstra E., Bracken R.M. Poor glycaemic control is associated with reduced exercise performance and oxygen economy during cardio-pulmonary exercise testing in people with type 1 diabetes. Diabetol. Metab. Syndr. 2017;9:93. doi: 10.1186/s13098-017-0294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moser O., Eckstein M.L., Mueller A., Birnbaumer P., Aberer F., Koehler G., Sourij C., Kojzar H., Pferschy P., Dietz P., et al. Pre-exercise blood glucose levels determine the amount of orally administered carbohydrates during physical exercise in individuals with type 1 diabetes—A randomized cross-over trial. Nutrients. 2019;11:1287. doi: 10.3390/nu11061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moser O., Tschakert G., Mueller A., Groeschl W., Eckstein M.L., Koehler G., Bracken R.M., Pieber T.R., Hofmann P. Different Heart Rate Patterns During Cardio-Pulmonary Exercise (CPX) Testing in Individuals With Type 1 Diabetes. Front. Endocrinol. 2018;9:1–8. doi: 10.3389/fendo.2018.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moser O., Eckstein M.L., McCarthy O., Deere R., Bain S.C., Haahr H.L., Zijlstra E., Heise T., Bracken R.M. Heart rate dynamics during cardio-pulmonary exercise testing are associated with glycemic control in individuals with type 1 diabetes. PLoS ONE. 2018;13:e0194750. doi: 10.1371/journal.pone.0194750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.MURRAY F.T., CAMERON D.F., VOGEL R.B., THOMAS R.G., WYSS H.U., ZAUNER C.W. The Pituitary-Testicular Axis at Rest and During Moderate Exercise in Males with Diabetes Mellitus and Normal Sexual Function. J. Androl. 1988;9:197–206. doi: 10.1002/j.1939-4640.1988.tb01034.x. [DOI] [PubMed] [Google Scholar]
- 85.Nadeau K.J., Regensteiner J.G., Bauer T.A., Brown M.S., Dorosz J.L., Hull A., Zeitler P., Draznin B., Reusch J.E.B. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J. Clin. Endocrinol. Metab. 2010;95:513–521. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nguyen T., Obeid J., Walker R.G., Krause M.P., Hawke T.J., Mcassey K., Vandermeulen J., Timmons B.W. Fitness and physical activity in youth with type 1 diabetes mellitus in good or poor glycemic control. Pediatr. Diabetes. 2015;16:48–57. doi: 10.1111/pedi.12117. [DOI] [PubMed] [Google Scholar]
- 87.Niranjan V., McBrayer D.G., Ramirez L.C., Raskin P., Hsia C.C.W. Glycemic control and cardiopulmonary function in patients with insulin- dependent diabetes mellitus. Am. J. Med. 1997;103:504–513. doi: 10.1016/S0002-9343(97)00251-9. [DOI] [PubMed] [Google Scholar]
- 88.Peltonen J.E., Koponen A.S., Pullinen K., Hägglund H., Aho J.M., Kyröläinen H., Tikkanen H.O. Alveolar gas exchange and tissue deoxygenation during exercise in type 1 diabetes patients and healthy controls. Respir. Physiol. Neurobiol. 2012;181:267–276. doi: 10.1016/j.resp.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 89.Peltoniemi P., Yki-Järvinen H., Oikonen V., Oksanen A., Takala T.O., Rönnemaa T., Erkinjuntti M., Knuuti M.J., Nuutila P. Resistance to exercise-induced increase in glucose uptake during hyperinsulinemia in insulin-resistant skeletal muscle of patients with type 1 diabetes. Diabetes. 2001;50:1371–1377. doi: 10.2337/diabetes.50.6.1371. [DOI] [PubMed] [Google Scholar]
- 90.Poortmans J.R., Saerens P., Edelman R., Vertongen F., Dorchy H. Influence of the degree of metabolic control on physical fitness in type 1 diabetic adolescents. Int. J. Sports Med. 1986;7:232–235. doi: 10.1055/s-2008-1025765. [DOI] [PubMed] [Google Scholar]
- 91.Raguso C.A., Coggan A.R., Gastaldelli A., Sidossis L.S., Edward J.B., III, Wolfe R.R. Lipid and Carbohydrate Metabolism in IDDM During Moderate and Intense Exercise. Diabetes. 1995;44:1066–1074. doi: 10.2337/diab.44.9.1066. [DOI] [PubMed] [Google Scholar]
- 92.Reddy R., Wittenberg A., Castle J.R., El Youssef J., Winters-Stone K., Gillingham M., Jacobs P.G. Effect of Aerobic and Resistance Exercise on Glycemic Control in Adults With Type 1 Diabetes. Can. J. Diabetes. 2019;43:406–414.e1. doi: 10.1016/j.jcjd.2018.08.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rigla M., Sanchez-Quesada J.L., Ordonez-Llanos J., Prat T., Caixas A., Jorba O., Serra J.R., De Leiva A., Perez A. Effect of physical exercise on lipoprotein(a) and low-density lipoprotein modifications in type 1 and type 2 diabetic patients. Metabolism. 2000;49:640–647. doi: 10.1016/S0026-0495(00)80041-4. [DOI] [PubMed] [Google Scholar]
- 94.Rigla M., Fontcuberta J., Mateo J., Caixàs A., Pou J.M., De Leiva A., Pérez A. Physical training decreases plasma thrombomodulin in Type I and Type II diabetic patients. Diabetologia. 2001;44:693–699. doi: 10.1007/s001250051677. [DOI] [PubMed] [Google Scholar]
- 95.Rissanen A.P.E., Tikkanen H.O., Koponen A.S., Aho J.M., Peltonen J.E. Central and peripheral cardiovascular impairments limit VO2peak in Type 1 diabetes. Med. Sci. Sports Exerc. 2015;47:223–230. doi: 10.1249/MSS.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 96.Rissanen A.-P., Tikkanen H.O., Koponen A.S., Jyrki M.A., Peltonen J.E. One-year unsupervised individualized exercise training intervention enhances cardiorespiratory fitness but not muscle deoxygenation or glycemic control in adults with type 1 diabetes. Appl. Physiol. Nutr. Metab. 2018;43:387–396. doi: 10.1139/apnm-2017-0222. [DOI] [PubMed] [Google Scholar]
- 97.Roberts T.J., Burns A.T., MacIsaac R.J., MacIsaac A.I., Prior D.L., Gerche A. La Diagnosis and significance of pulmonary microvascular disease in diabetes. Diabetes Care. 2018;41:854–861. doi: 10.2337/dc17-1904. [DOI] [PubMed] [Google Scholar]
- 98.Roberts T.J., Burns A.T., MacIsaac R.J., MacIsaac A.I., Prior D.L., La Gerche A. Exercise capacity in diabetes mellitus is predicted by activity status and cardiac size rather than cardiac function: A case control study. Cardiovasc. Diabetol. 2018;17:1–14. doi: 10.1186/s12933-018-0688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roberts T.J., Barros-Murphy J.F., Burns A.T., MacIsaac R.J., MacIsaac A.I., Prior D.L., La Gerche A. Reduced Exercise Capacity in Diabetes Mellitus Is Not Associated with Impaired Deformation or Twist. J. Am. Soc. Echocardiogr. 2020;33:481–489. doi: 10.1016/j.echo.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 100.Robitaille M., Dubé M.C., Weisnagel S.J., Prud’homme D., Massicotte D., Péronnet F., Lavoie C. Substrate source utilization during moderate intensity exercise with glucose ingestion in Type 1 diabetic patients. J. Appl. Physiol. 2007;103:119–124. doi: 10.1152/japplphysiol.01462.2006. [DOI] [PubMed] [Google Scholar]
- 101.Roche D.M., Edmunds S., Cable T., Didi M., Stratton G. Skin microvascular reactivity in children and adolescents with type 1 diabetes in relation to levels of physical activity and aerobic fitness. Pediatr. Exerc. Sci. 2008;20:426–438. doi: 10.1123/pes.20.4.426. [DOI] [PubMed] [Google Scholar]
- 102.Rowland T.W., Martha P.M., Jr., Reiter E.O., Cunningham L.N. The Influence of Diabetes Mellitus on Cardiovascular Function in Children and Adolescents. Int. J. Sports Med. 1992;13:431–435. doi: 10.1055/s-2007-1021293. [DOI] [PubMed] [Google Scholar]
- 103.Roy-Fleming A., Taleb N., Messier V., Suppère C., Cameli C., Elbekri S., Smaoui M.R., Ladouceur M., Legault L., Rabasa-Lhoret R. Timing of insulin basal rate reduction to reduce hypoglycemia during late post-prandial exercise in adults with type 1 diabetes using insulin pump therapy: A randomized crossover trial. Diabetes Metab. 2019;45:294–300. doi: 10.1016/j.diabet.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 104.Sandoval D.A., Aftab Guy D.L., Richardson M.A., Ertl A.C., Davis S.N. Effects of Low and Moderate antecedent exercise on counterregulatory responses to subsequent hypoglycemia in type 1 diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2004;290 doi: 10.1152/ajpendo.00283.2005. [DOI] [PubMed] [Google Scholar]
- 105.Schneider S.H., Khachadurian A.K., Amorosa L.F., Clemow L., Ruderman N.B. Ten-Year Experience With an Exercise-Based Outpatient Life-Style Modification Program in the Treatment of Diabetes Mellitus. Diabetes Care. 1992;15:1800–1810. doi: 10.2337/diacare.15.11.1800. [DOI] [PubMed] [Google Scholar]
- 106.Seeger J.P.H., Thijssen D.H.J., Noordam K., Cranen M.E.C., Hopman M.T.E., Nijhuis-Van Der Sanden M.W.G. Exercise training improves physical fitness and vascular function in children with type 1 diabetes. Diabetes, Obes. Metab. 2011;13:382–384. doi: 10.1111/j.1463-1326.2011.01361.x. [DOI] [PubMed] [Google Scholar]
- 107.Shetty V.B., Fournier P.A., Davey R.J., Retterath A.J., Paramalingam N., Roby H.C., Davis E.A., Jones T.W. The time lag prior to the rise in glucose requirements to maintain stable glycaemia during moderate exercise in a fasted insulinaemic state is of short duration and unaffected by the level at which glycaemia is maintained in Type 1 diabetes. Diabet. Med. 2018;35:1404–1411. doi: 10.1111/dme.13771. [DOI] [PubMed] [Google Scholar]
- 108.Singhvi A., Tansey M., Janz K., Zimmerman M., Tsalikian E. Aerobic Fitness and Glycemic Variability in Adolescents with Type 1 Diabetes. Endocr. Pract. 2014;20:566–570. doi: 10.4158/EP13211.OR. [DOI] [PubMed] [Google Scholar]
- 109.Stettler C., Jenni S., Allemann S., Steiner R., Hoppeler H., Trepp R., Christ E.R., Zwahlen M., Diem P. Exercise capacity in subjects with type 1 diabetes mellitus in eu- and hyperglycaemia. Diabetes Metab. Res. Rev. 2006;22:300–306. doi: 10.1002/dmrr.608. [DOI] [PubMed] [Google Scholar]
- 110.Stewart C.J., Nelson A., Campbell M.D., Walker M., Stevenson E.J., Shaw J.A., Cummings S.P., West D.J. Gut microbiota of Type 1 diabetes patients with good glycaemic control and high physical fitness is similar to people without diabetes: An observational study. Diabet. Med. 2017;34:127–134. doi: 10.1111/dme.13140. [DOI] [PubMed] [Google Scholar]
- 111.Tagougui S., Leclair E., Fontaine P., Matran R., Marais G., Aucouturier J., Descatoire A., Vambergue A., Oussaidene K., Baquet G., et al. Muscle oxygen supply impairment during exercise in poorly controlled Type 1 diabetes. Med. Sci. Sports Exerc. 2015;47:231–239. doi: 10.1249/MSS.0000000000000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tagougui S., Fontaine P., Leclair E., Aucouturier J., Matran R., Oussaidene K., Descatoire A., Prieur F., Mucci P., Vambergue A., et al. Regional cerebral hemodynamic response to incremental exercise is blunted in poorly controlled patients with uncomplicated type 1 diabetes. Diabetes Care. 2015;38:858–867. doi: 10.2337/dc14-1792. [DOI] [PubMed] [Google Scholar]
- 113.Tagougui S., Goulet-Gelinas L., Taleb N., Messier V., Suppere C., Rabasa-Lhoret R. Association Between Body Composition and Blood Glucose During Exercise and Recovery in Adolescent and Adult Patients With Type 1 Diabetes. Can. J. Diabetes. 2020;44:192–195. doi: 10.1016/j.jcjd.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 114.Tonoli C., Heyman E., Buyse L., Roelands B., Piacentini M.F., Bailey S., Pattyn N., Berthoin S., Meeusen R. Neurotrophins and cognitive functions in T1D compared with healthy controls: Effects of a high-intensity exercise. Appl. Physiol. Nutr. Metab. 2014;40:20–27. doi: 10.1139/apnm-2014-0098. [DOI] [PubMed] [Google Scholar]
- 115.Trigona B., Aggoun Y., Maggio A., Martin X.E., Marchand L.M., Beghetti M., Farpour-Lambert N.J. Preclinical noninvasive markers of atherosclerosis in children and adolescents with type 1 diabetes are influenced by physical activity. J. Pediatr. 2010;157:533–539. doi: 10.1016/j.jpeds.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 116.Tuominen J.A., Ebeling P., Vuorinen-Markkola H., Koivisto V.A. Post-marathon paradox in IDDM: Unchanged insulin sensitivity in spite of glycogen depletion. Diabet. Med. 1997;14:301–308. doi: 10.1002/(SICI)1096-9136(199704)14:4<301::AID-DIA346>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 117.Turinese I., Marinelli P., Bonini M., Rossetti M., Statuto G., Filardi T., Paris A., Lenzi A., Morano S., Palange P. Metabolic and cardiovascular response to exercise in patients with type 1 diabetes. J. Endocrinol. Invest. 2017;40:999–1005. doi: 10.1007/s40618-017-0670-6. [DOI] [PubMed] [Google Scholar]
- 118.Tuttle K.R., Marker J.C., Dalsky G.P., Schwartz N.S., Shah S.D., Clutter W.E., Holloszy J.O., Cryer P.E. Glucagon, not insulin, may play a secondary role in defense against hypoglycemia during exercise. Am. J. Physiol. Endocrinol. Metab. 1988;254:713–719. doi: 10.1152/ajpendo.1988.254.6.E713. [DOI] [PubMed] [Google Scholar]
- 119.Valletta J.J., Chipperfield A.J., Clough G.F., Byrne C.D. Daily energy expenditure, cardiorespiratory fitness and glycaemic control in people with type 1 diabetes. PLoS ONE. 2014;9:e97534. doi: 10.1371/journal.pone.0097534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Veves A., Saouaf R., Donaghue V.M., Mullooly C.A., Kistler J.A., Giurini J.M., Horton E.S., Fielding R.A. Aerobic exercise capacity remains normal despite impaired endothelial function in the micro- and macrocirculation of physically active IDDM patients. Diabetes. 1997;46:1846–1852. doi: 10.2337/diab.46.11.1846. [DOI] [PubMed] [Google Scholar]
- 121.Waclawovsky G., Umpierre D., Figueira F.R., De Lima E.S., Alegretti A.P., Schneider L., Matte U.S., Rodrigues T.C., Schaan B.D. Exercise on progenitor cells in healthy subjects and patients with type 1 diabetes. Med. Sci. Sports Exerc. 2016;48:190–199. doi: 10.1249/MSS.0000000000000764. [DOI] [PubMed] [Google Scholar]
- 122.Wallberg-Henriksson H., Gunnarsson R., Henriksson J., DeFronzo R., Felig P., Ostman J., Wahren J. Increased peripheral insulin sensitivity and muscle mitochondrial enzymes but unchanged blood glucose control in type I diabetics after physical training. Diabetes. 1982;31:1044–1050. doi: 10.2337/diacare.31.12.1044. [DOI] [PubMed] [Google Scholar]
- 123.Wallberg-Henriksson H., Gunnarsson R., Rössner S., Wahren J. Long-term physical training in female Type 1 (Insulin-dependent) diabetic patients: Absence of significant effect on glycaemic control and lipoprotein levels. Diabetologia. 1986;1:53–57. doi: 10.1007/BF02427281. [DOI] [PubMed] [Google Scholar]
- 124.Wanke T., Formanek D., Auinger M., Zwick H., Irsigler K. Pulmonary Gas Exchange and Oxygen Uptake During Exercise in Patients with Type 1 Diabetes Mellitus. Diabet. Med. 1992;9:252–257. doi: 10.1111/j.1464-5491.1992.tb01771.x. [DOI] [PubMed] [Google Scholar]
- 125.Wilson L.C., Peebles K.C., Hoye N.A., Manning P., Sheat C., Williams M.J.A., Wilkins G.T., Wilson G.A., Baldi J.C. Resting heart rate variability and exercise capacity in Type 1 diabetes. Physiol. Rep. 2017;5:1–12. doi: 10.14814/phy2.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yardley J.E., Kenny G.P., Perkins B.A., Riddell M.C., Malcolm J., Boulay P., Khandwala F., Sigal R.J. Effects of performing resistance exercise before versus after aerobic exercise on glycemia in type 1 diabetes. Diabetes Care. 2012;35:669–675. doi: 10.2337/dc11-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yardley J.E., Kenny G.P., Perkins B.A., Riddell M.C., Balaa N., Malcolm J., Boulay P., Khandwala F., Sigal R.J. Resistance versus aerobic exercise. Diabetes Care. 2013;36:537–542. doi: 10.2337/dc12-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yardley J.E., Sigal R.J., Kenny G.P., Riddell M.C., Lovblom L.E., Perkins B.A. Point Accuracy of interstitial continuous glucose monitoring during exercise in type 1 diabetes. Diabetes Technol. Ther. 2013;15:46–49. doi: 10.1089/dia.2012.0182. [DOI] [PubMed] [Google Scholar]
- 129.Yardley J.E., Iscoe K.E., Sigal R.J., Kenny G.P., Perkins B.A., Riddell M.C. Insulin Pump Therapy is associated with Less Post-Exercise Hyperglycemia than multiple daily injections: An observational study of physically active type 1 diabetes patients. Diabetes Technol. Ther. 2013;15:84–88. doi: 10.1089/dia.2012.0168. [DOI] [PubMed] [Google Scholar]
- 130.Zaharieva D.P., McGaugh S., Pooni R., Vienneau T., Ly T., Riddell M.C. Improved Open-Loop Glucose Control With Basal Insulin Reduction 90 Minutes Before Aerobic Exercise in Patients With Type 1 Diabetes on Continuous Subcutaneous Insulin Infusion. Diabetes Care. 2019;42:824–831. doi: 10.2337/dc18-2204. [DOI] [PubMed] [Google Scholar]
- 131.Zaharieva D.P., Miadovnik L.A., Rowan C.P., Gumieniak R.J., Jamnik V.K., Riddell M.C. Effects of acute caffeine supplementation on reducing exercise-associated hypoglycaemia in individuals with Type 1 diabetes mellitus. Diabet. Med. 2016;33:488–496. doi: 10.1111/dme.12857. [DOI] [PubMed] [Google Scholar]
- 132.Zaharieva D., Yavelberg L., Jamnik V., Cinar A., Turksoy K., Riddell M.C. The effects of basal insulin suspension at the start of exercise on blood glucose levels during continuous versus circuit-based exercise in individuals with type 1 diabetes on continuous subcutaneous insulin infusion. Diabetes Technol. Ther. 2017;19:370–378. doi: 10.1089/dia.2017.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zebrowska A., Hall B., Kochanska-Dziurowicz A., Janikowska G. The effect of high intensity physical exercise and hypoxia on glycemia, angiogenic biomarkers and cardiorespiratory function in patients with type 1 diabetes. Adv. Clin. Exp. Med. 2018;27:207–216. doi: 10.17219/acem/66354. [DOI] [PubMed] [Google Scholar]
- 134.Kennedy A., Nirantharakumar K., Chimen M., Pang T.T., Hemming K., Andrews R.C., Narendran P. Does Exercise Improve Glycaemic Control in Type 1 Diabetes? A Systematic Review and Meta-Analysis. PLoS ONE. 2013;8:e58861. doi: 10.1371/journal.pone.0058861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yardley J., Mollard R., MacIntosh A., MacMillan F., Wicklow B., Berard L., Hurd C., Marks S., McGavock J. Vigorous intensity exercise for glycemic control in patients with type 1 diabetes. Can. J. Diabetes. 2013;37:427–432. doi: 10.1016/j.jcjd.2013.08.269. [DOI] [PubMed] [Google Scholar]
- 136.Moser O., Riddell M.C., Eckstein M.L., Adolfsson P., Rabasa-Lhoret R., van den Boom L., Gillard P., Nørgaard K., Oliver N.S., Zaharieva D.P., et al. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: Position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA) Diabetologia. 2020;63:2501–2520. doi: 10.1007/s00125-020-05263-9. [DOI] [PubMed] [Google Scholar]
- 137.Bohn B., Herbst A., Pfeifer M., Krakow D., Zimny S., Kopp F., Melmer A., Steinacker J.M., Holl R.W. Impact of physical activity on glycemic control and prevalence of cardiovascular risk factors in adults with type 1 diabetes: A cross-sectional multicenter study of 18,028 patients. Diabetes Care. 2015;38:1536–1543. doi: 10.2337/dc15-0030. [DOI] [PubMed] [Google Scholar]
- 138.King K.M., Jaggers J.R., Della L.J., McKay T., Watson S., Kozerski A.E., Hartson K.R., Wintergerst K.A. Association between Physical Activity and Sport Participation on Hemoglobin A1c Among Children and Adolescents with Type 1 Diabetes. Int. J. Environ. Res. Public Health. 2021;18:7490. doi: 10.3390/ijerph18147490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Laukkanen J.A., Zaccardi F., Khan H., Kurl S., Jae S.Y., Rauramaa R. Long-term Change in Cardiorespiratory Fitness and All-Cause Mortality: A Population-Based Follow-up Study. Mayo Clin. Proc. 2016;91:1183–1188. doi: 10.1016/j.mayocp.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 140.Strasser B. Survival of the fittest VO sub 2 sub max a key predictor of longevity. Front. Biosci. 2018;23:4657. doi: 10.2741/4657. [DOI] [PubMed] [Google Scholar]
- 141.Clements M.A., Foster N.C., Maahs D.M., Schatz D.A., Olson B.A., Tsalikian E., Lee J.M., Burt-Solorzano C.M., Tamborlane W.V., Chen V., et al. Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatr. Diabetes. 2016;17:327–336. doi: 10.1111/pedi.12295. [DOI] [PubMed] [Google Scholar]
- 142.Rodrigues A.N., Perez A.J., Carletti L., Bissoli N.S., Abreu G.R. Maximum oxygen uptake in adolescents as measured by cardiopulmonary exercise testing: A classification proposal. J. Pediatr. 2006;82:426–430. doi: 10.2223/JPED.1533. [DOI] [PubMed] [Google Scholar]
- 143.King K.M., McKay T., Thrasher B.J., Wintergerst K.A. Maximal Oxygen Uptake, VO2 Max, Testing Effect on Blood Glucose Level in Adolescents with Type 1 Diabetes Mellitus. Int. J. Environ. Res. Public Health. 2022;19:5543. doi: 10.3390/ijerph19095543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Karakelides H., Asmann Y.W., Bigelow M.L., Short K.R., Dhatariya K., Coenen-Schimke J., Kahl J., Mukhopadhyay D., Nair K.S. Effect of Insulin Deprivation on Muscle Mitochondrial ATP Production and Gene Transcript Levels in Type 1 Diabetic Subjects. Diabetes. 2007;56:2683–2689. doi: 10.2337/db07-0378. [DOI] [PubMed] [Google Scholar]
- 145.Krause M.P., Riddell M.C., Hawke T.J. Effects of type 1 diabetes mellitus on skeletal muscle: Clinical observations and physiological mechanisms. Pediatr. Diabetes. 2011;12:345–364. doi: 10.1111/j.1399-5448.2010.00699.x. [DOI] [PubMed] [Google Scholar]
- 146.Radin M.S. Pitfalls in Hemoglobin A1c Measurement: When Results may be Misleading. J. Gen. Intern. Med. 2013;29:388–394. doi: 10.1007/s11606-013-2595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.