Abstract
Mechanisms of silicosis, caused by the inhalation of silica are still unclear, and the effect of sex on silicosis has rarely been reported. The purpose of this study was to investigate whether sex affects the silicotic lesions and the progressive fibrotic responses in silicosis. Our study showed that sex had no significant effect on the area of silicon nodules and the collagen deposition after a one-time bronchial perfusion of silica. Immunohistochemical staining showed that CD68 and the transforming growth factor-β1 (TGF-β1) were positive in male and female silicotic mice. In addition, the western blot results showed that the fibrosis-related factors type I collagen (COL I), α-smooth muscle actin (α-SMA), vimentin, TGF-β1, p-SMAD2/3, inflammatory-related factors interleukin 6 (IL 6), interleukin 1β (IL 1β), and senescence-related factors p16 and p21 were up-regulated in silicotic mice and there was no difference between female or male mice exposed to silica. The expression of TGF-β1, p-SMAD2/3, p16, and p21 were downregulated in the early stage of female silicotic mice, compared to the males. Thus, despite differences in the expression of certain factors, there was no overall difference in the progressive fibrosis between female and male mice in silicosis. These results thus provide a new perspective for studying the pathological development of silicosis.
Keywords: silicosis, mouse sex, collagen, inflammatory response, senescence, fibrosis
1. Introduction
Pneumoconiosis, caused by the inhalation of silica (SiO2), is the most common occupational disease in China, accounting for about 80% of the total number of occupational diseases reported every year [1]. However, due to the low coverage of occupational health examinations and the strict diagnostic criteria for pneumoconiosis, the number of cases is underestimated [2]. The mechanisms of silicosis are not very clear yet, and thus effective treatments are still lacking, relying on lung transplantation [3].
The pathogenesis of silicosis has been addressed in vivo and in vitro for many years [4,5,6,7] Factors, such as inflammatory response, transforming growth factor-β (TGF-β) signaling, and senescence-related factors [4,5,7,8,9], play important roles in the progression of experimental silicosis. Most studies performed were on male mice or rats.
In several studies related to the occupational environments dominated by men, often there is no distinction by sex or women are excluded from the analysis. It is this chronic neglect that has led to the default choice of male subjects in most studies. In recent descriptions, nearly 100% of coal workers with advanced pneumoconiosis are men [10,11] Similarly, in large epidemiologic surveillance programs for silicosis across the United States, more than 95% of cases were found in men [12]. From a few available epidemiological data on the sex distribution of silicosis patients, it is observed that even if the number of female patients is small, it is undeniable that there is a female group among the silicosis patients. Thus, it is not possible to theorize that either men or women are more susceptible to the effects of silica. Therefore, to provide a theoretical basis for the pathological study and the clinical treatment of silicosis, it is necessary to investigate the effect of the sex differences on silicotic animal models.
Sex has also been reported as a critical factor in determining the susceptibility toward various agents that cause acute or chronic respiratory inflammation in human pulmonary diseases [13,14]. A study about the sex differences in the incidence and outcomes of idiopathic pulmonary fibrosis (IPF) patients showed that in elderly patients, the incidence of IPF was higher among men than women [15]. Similarly, in murine models of chemically induced pulmonary fibrosis, female mice were protected from the chronic complications with a single exposure to either hydrochloric acid or nitrogen mustard [16]. In a similar study, Dorothy et al. found that male mice were more susceptible to a single acute or repeated sub-chronic exposure to nickel nanoparticles, in the absence or presence of lipopolysaccharide [17]. All of these studies thus suggested that sex difference seems to play a significant role in the progression of pulmonary diseases. Based on the existing research, the present study determined the effect of sex on experimental silicosis mice and provided a new perspective for studying the pathological development of silicosis in the future.
2. Results
2.1. Effects of Sex on the Silicotic Nodules and Pulmonary Functions
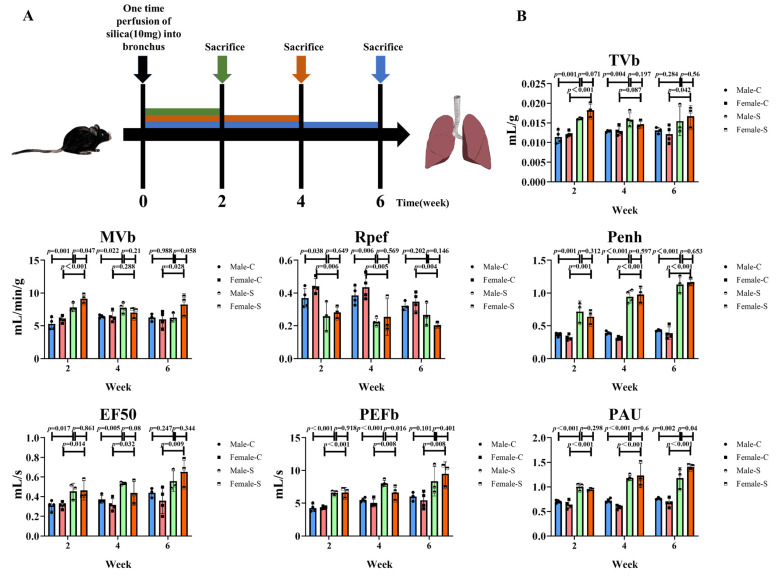
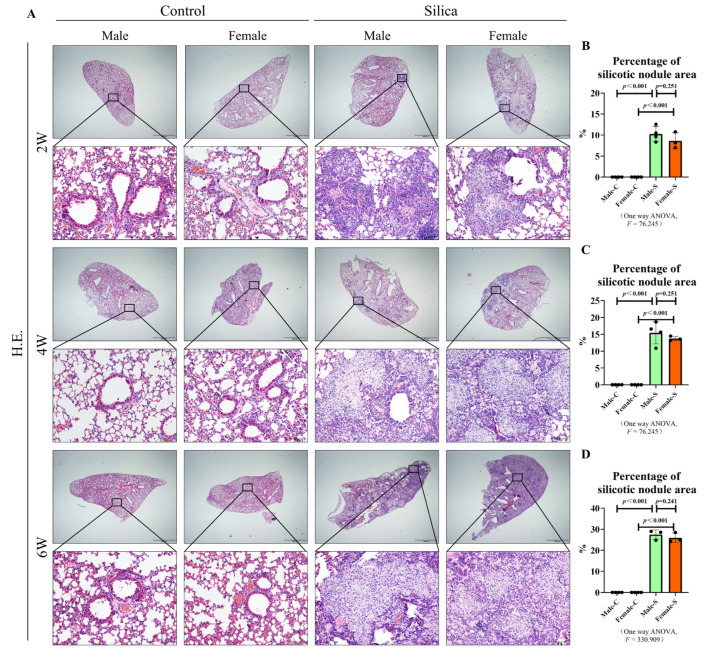
To determine the effects of sex on the silicotic nodules, the silicosis models were established by injecting silica into the trachea of male and female mice. The mice were further sacrificed at different time points (Figure 1A). The death rate of the mice was 3.3%, and the average weight of the mice in the control group increased slightly with the prolongation of time (male: 20-22-23-25 g; female: 16-17-20-22 g), while the average weight of mice in the silicosis group decreased significantly after two weeks of modeling (male: 20-18 g; female: 16-15 g), and then increased slightly (male: 20-18-21-23 g; female: 16-15-17-20 g). The results showed that sex had no significant effect on the multiple indexes of the pulmonary function at most time points (Figure 1B). The hematoxylin and eosin (H&E) staining showed a small number of minute silicotic nodules consisting of macrophages around the bronchus. The alveolus septum was thickened after two weeks of the silica stimulation in both male and female mice. By the fourth week, the volume and number of silicotic nodules increased, and some silicotic nodules were found to be merged. By six weeks, silicotic nodules were widely distributed in the lung tissues. The normal alveolus structure was destroyed and replaced by large numbers of silica nodules containing collagen. However, there was no significant difference in the area of the silicotic nodules between male and female mice, at all time points (Figure 2A–D).
Figure 1.
Indexes of the pulmonary function of male and female mice exposed to silica in two, four, and six weeks, and the control groups. (A) Schematic diagram of a silicotic model establishment and execution. (B) Detection indexes of a lung function, n = 5 per group.
Figure 2.
H&E staining of male and female mice exposed to silica in two, four, and six weeks, and the control groups. (A) H&E staining of the lung tissues in mice exposed to silica (scale bar = 2000 µm). (B–D) Statistical analysis of the H&E staining, n = 5 per group ((B)—2W, (C)—4W, (D)—6W).
2.2. Effects of Sex on the Collagen Deposition in Silicosis
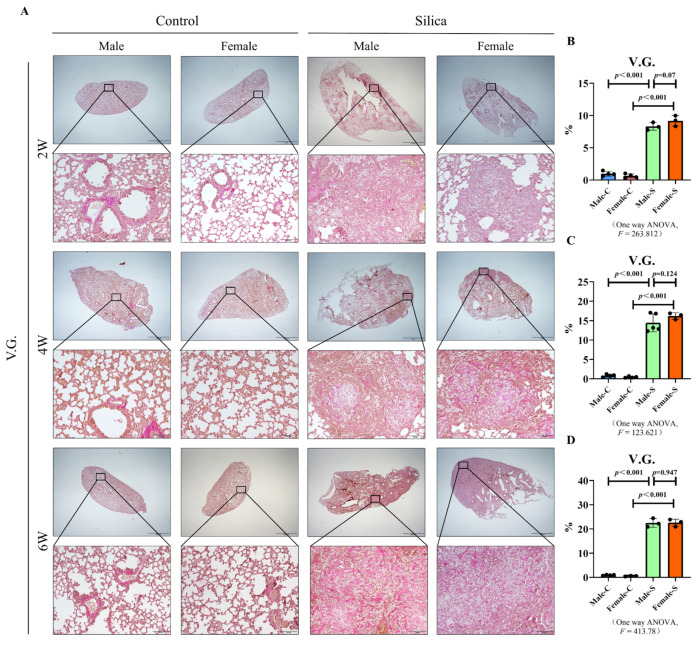
The deposition of collagen is one of the main characteristics of silicosis. Van Gieson staining (V.G. staining) showed only a small amount of collagen around the blood vessels in the lung tissues of the male and female control groups. However, a huge amount of red-stained collagen was deposited in the silicotic nodules of the silica-exposed mice. In addition, we also found that the degree of collagen in the silicotic nodules increased in a time-dependent manner in both male and female silicosis groups. Fibrosis-related factors, type I collagen (COL I), and α-smooth muscle actin (α-SMA) [18,19] were analyzed using the western blot. No difference was observed in the expression of COL I and α-SMA between the female and male mice at two, four, or six weeks (Figure 3). These results indicated no difference in the collagen content between the female and male mice (Figure 3 and Figure 4).
Figure 3.
V.G. staining of the male and female mice exposed to silica in two, four, and six weeks, and the control groups. (A) V.G. staining of the lung tissues in mice exposed to silica (scale bar = 2000 and 100 µm). (B–D) Statistical analysis of the V.G. staining, n = 5 per group ((B)—2W, (C)—4W, (D)—6W).
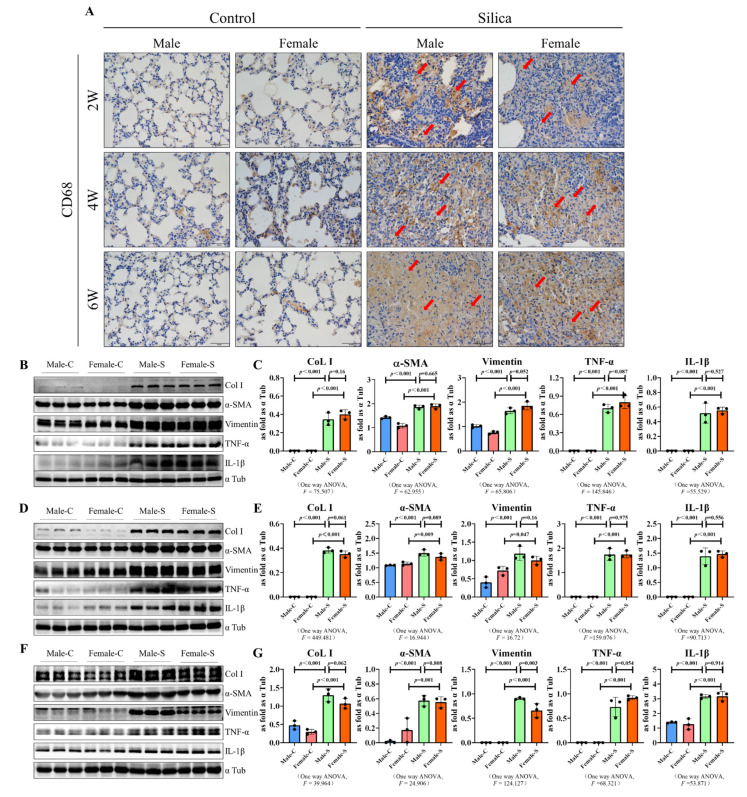
Figure 4.
Western blot of CD68, COL I, vimentin, and α-SMA in male and female mice exposed to silica for two, four, six weeks, and the control groups. (A) Immunohistochemical staining was used to detect the expression of CD68 in the mouse lung tissues, arrow: CD68-positive cells (brown) (scale bar = 50 µm). (B–G) Expression levels of Col I, α-SMA, vimentin, IL 6 and IL 1β in the lungs of mice were measured using the western blot. Data are presented as the mean ± SD, n = 5 per group ((B,C)—2W, (D,E)—4W, (F,G)—6W).
2.3. Effects of Sex on the Inflammation Response in Silicosis
The immunohistochemical results showed that CD68, a recognized marker of the macrophages, was highly expressed in both silicotic male and female groups. These results indicated that silica activated the macrophages in both female and male mice. As evidence, the vimentin expression was also significantly increased in the silicosis model group. The expression of the inflammation-related factors interleukin 6 (IL 6) and interleukin 1β (IL 1β) were significantly up-regulated in the silicosis model group, but were consistent with the collagen deposition. There was no significant difference in the expression of these factors in the female and male mice, with respect to the markers or different time points (Figure 4).
2.4. Effects of Sex on the TGF-β Signal-Related Factors and Senescence-Related Factors in Silicosis
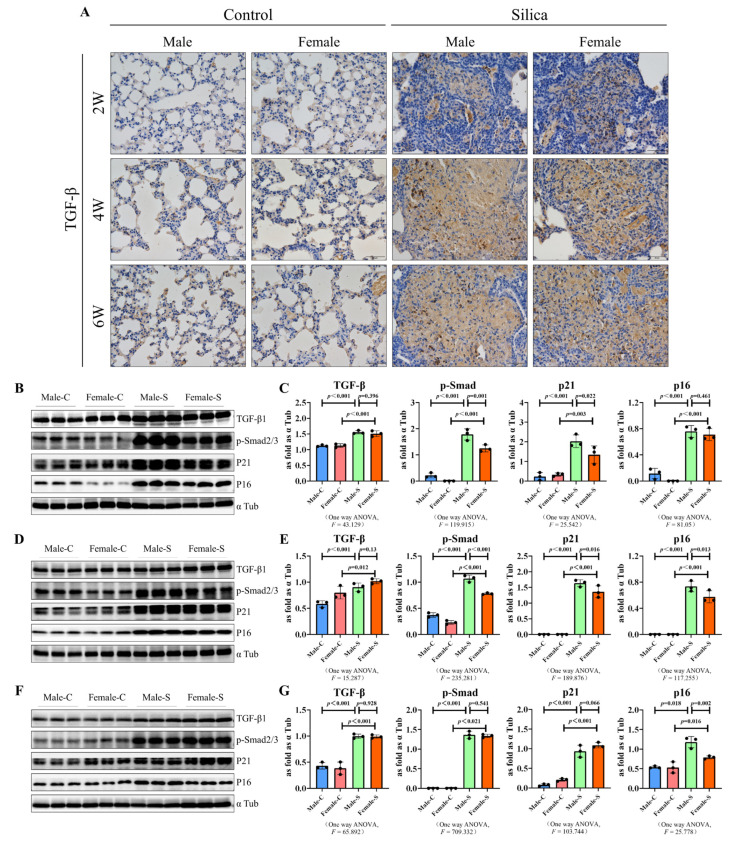
Activation of the TGF-β signaling is one of the driving factors of pulmonary fibrosis [20], thus the effects of sex were also studied on the TGF-β signal-related factors in silicosis mice. The immunohistochemical results showed that TGF-β1 was highly expressed in the fibrotic lung tissues and there was no different between the males and females (Figure 5A). Even though the p-SMAD2/3 protein levels were significantly decreased at two and four weeks in females, than in the male mice, at six weeks, there was no difference in the expression of p-SMAD2/3 between the female mice. The role of the cellular senescence in the silicosis progression was described previously [4], we also detected the protein expression of senescence-related factors. Furthermore, the expression of p21 was slightly downregulated at two and four weeks. Interestingly, the p16 levels were significantly downregulated in the four and six week groups (Figure 5B–G).
Figure 5.
Western blots of TGF-β1, p-SMAD2/3, p21, and p16 in the male and female mice exposed to silica for two, four, six weeks, and the control groups. (A) Immunohistochemical staining was used to detect the expression of TGF-β (brown) in the mouse lung tissues (scale bar = 50 µm). (B–G) Expression levels of TGF-β1, p-SMAD2/3, p21, and p16 in the lungs of mice were measured using the western blot. Data are presented as the mean ± SD, n = 5 per group ((B,C)—2W, (D,E)—4W, (F,G)—6W).
3. Discussion
This study aimed to determine the effects of the sex differences in the lung responses after a single silica exposure, and the mechanisms of the differential susceptibility between sexes in silicosis. The cause of silicosis is fairly clear—the inhalation of quartz particles. The conventional wisdom is that only male miners would suffer from silicosis, with a perception that there are more men in industrial and labor workforces [21]. However, with deepening understanding of pulmonary diseases, the incidence of silicosis among jeans sandblasters and porcelain grinders, involving many women, have come to light [22,23]. Additionally, some areas with a high incidence of sandstorms, where people of both sexes and ages are chronically exposed to quartz particles and air pollution, are associated with the risk of developing or exacerbating IPF [24]. Based on the above data, it became necessary to study the effects of the sex differences in silicosis. To our knowledge, this is the first study to investigate the effect of sex differences on the lung’s response toward SiO2, in an animal model.
We observed that at all time points, the silica nodule formation and collagen deposition occurred in both female and male mice. However, consistent with our previous findings, the content of collagen in lung tissues, in all the groups, increased gradually with the exposure to silica and there was no difference between the male and female silicotic mice. Although it has been reported that the collagen content of both male and female mice gradually increases with age [25], our results clearly show that the increase of collagen in the lungs of mice in the model group is mainly caused by the stimulation of silica. Epidemiological results showed that the prevalence of the interstitial lung disease in children under two-years-old was much higher in males than in females [26]. These results may be attributed to their narrower airways, leading to a longer persistence of inhaled substances in the periphery of the male lungs. A more prolonged exposure may elicit a stronger adverse response and subsequent chronic disease [27]. On the contrary, another study showed that in rats, females were more sensitive to the bleomycin stimulation, compared with males. Female rats may have an exaggerated response to lung injury, relative to male rats, because of the female sex hormones, which have a direct fibrogenic activity on the lung fibroblasts [28]. However, studies in mice revealed that there was either no significant difference or greater male responsiveness in the bleomycin-induced pulmonary fibrosis [29]. The differential response to a bleomycin-induced pulmonary fibrosis, may be due to an inheritable trait controlled by a few genetic loci, and that the X-linked factor may be associated with the fibrosis phenotype [30]. In the present study, we found no difference in the silicotic nodule area and the collagen deposition, consistent with the results of the bleomycin-induced mice studies. Considering these results, we believed that the effect of sex on the formation of silicotic nodules was not strong enough to influence the area of silicotic nodules and the degree of collagen deposition. The pulmonary function of silicosis patients is significantly impaired [31], and the non-invasive whole-body plethysmograph system can obtain the pulmonary function data of mice well [32]. With reference to the previous literature [33], in the present study, due to the increased restrictive ventilatory dysfunction and airway resistance, the indexes of Penh, EF 50, PAU and PEFb were higher in silicotic mice. Moreover, between male and female silicotic mice, however, there was no difference in the pulmonary function, on the whole.
The molecular mechanisms of the silica-induced inflammation and fibrosis, have not been completely understood yet. However, there has been a generally accepted concept supporting the contribution of inflammation in the development of fibrosis and the autoimmune responses, due to silica inhalation [34,35]. Our previous studies also indicated that as an important part of the body’s defense against external damage, the inflammatory response also plays an important role in the progression of silicosis [5,7]. Normally, sex hormone receptors are typically expressed intracellularly, however, in immune cells, the expression of the sex hormones has also been found on the cell surface where they interact as part of the immunological synapse, during the antigen presentation [36]. Most studies have not taken these factors into account when summarizing the effects of sex hormones on immune cells or their responses [37]. Moreover, in most fields of silicosis and inflammation-related studies, the sex factor was not taken into account, they directly used male rats or mice [38]. However, based on our results, the oversight did not affect the final results, and there were no significant differences in the expression of the inflammation-related factors in female and male mice. As evidence, in a study by Du et al., female mice were used to study the inflammatory response in the silicosis progression [39]. It is worth noting that another study figured out that silica-exposed males had more lung inflammation, bronchoalveolar lavage fluid cells, IL-6, and autoantibodies [40]. The reason for this phenomenon may be the differences in mouse species.
TGF-β1 drives the activation and functions of the fibroblasts and myofibroblasts to mediate fibrosis [41,42,43,44]. The present study showed that the expression of TGF-β1 was slightly upregulated in female and male mice exposed to silica, consistent with the previous research. The downstream factors of TGF-β1 and SMAD2/3 were also upregulated. Different from the sex chromosomes, which alter the intrinsic nature of cells, hormones are extrinsic factors that coordinate the cell behavior on a physiological scale. Particularly, the sex hormones directly regulate the members of the TGF-β signaling pathway [45]. Researchers have found that estrogen inhibits the TGF-β signaling by stimulating the SMAD2/3 protein degradation [46]. Our experimental results were a little bit inconsistent with this study. Compared with male mice, the expression of p-SMAD2/3 was downregulated in the female mice at two and four weeks, however, at six weeks, there was no difference between the expression of TGF-β1 in the females and males. A report on the diabetic nephropathy suggested that estradiol had an inhibitory effect on TGFBR1, however, there were no significant sex differences with regard to the renal function and fibrosis [47], consistent with our data. Another study found that the most important role of estradiol is disrupting the TGF-β signaling, rather than regulating its expression [48]. It is believed that estrogen has an inhibitory effect on the TGF-β1 expression, however, Nadja et al. showed that, during the progression of fibrosis, the estrogen induced the balance or imbalance between pro-fibrotic and anti-fibrotic signaling [47]. Therefore, whether estrogen plays a protective role in the progression of fibrosis through the TGF β signal pathway remains to be determined, in the present study, the expression of TGF-β1 and p-SMAD2/3 of female mice and male silicosis mice was ultimately not different.
Clinical follow-up studies have shown that long-term estrogen deficiency in women with ovariectomies as young adults would experience accelerated senescence phenotypes, once they are older [49]. Another research indicated that an estrogen deficiency and cellular senescence represented independent mechanisms in the pathogenesis of osteoporosis [50]. Yu et al. first identified the upregulation of the p21 in the osteocytic-rich area of ovariectomized mice [51]. Our previous findings showed that the senescence-related factors were up-regulated in silicosis mice [3]. In the present study, we found that, as compared to the two and four weeks, the expression of p16 was significantly down-regulated at six weeks in silicotic female mice. For the order of the differential expression of p21 and p16, it has been proposed that the effect of p21CIP1 may be limited to the onset of senescence, whereas p16INK4a maintains a durable growth arrest. Although induction of p21CIP1 is important for the initiation of senescence, its expression does not necessarily persist in senescent cells [52]. Our results suggested that male silicotic mice were more likely to show a cellular senescence than female mice.
Research in occupational pulmonary diseases has focused on men’s health, which introduced challenges and potential biases in characterizing women’s risks in specific workplaces and diagnostic delays [53]. In recent years, some researchers have also turned their attention to women with occupational lung disease [54,55]. Therefore, this paper focused on the effects of the sex differences in silicotic mice, although a detailed understanding of the disease processes will only be possible when the effect of sex on the signal crosstalk is elucidated. Our results showed that part of the classical fibrosis-related pathways were slightly downregulated in female silicotic mice, compared with males, but the ultimate degree of fibrosis did not differ between them.
4. Materials and Methods
4.1. Materials
Silica (s5631; Sigma–Aldrich, St. Louis, MO, USA), type I collagen (CoL I) (ab34710; Abcam, Cambridge, UK), α-smooth muscle actin (α-SMA) (ab5694; Abcam, Cambridge, UK), vimentin (ab92547, Abcam, Cambridge, UK), TGF-β1(ARG56429, Arigo, Shanghai, China), p-SMAD2/3(8828s, Cell Signaling, Massachusetts, USA), p21(ab109520, Abcam, Cambridge, UK), p16(A0262, Abclonal, Wuhan, China), IL-1β (DF6251; Affinity Biosciences, Cincinnati, OH, USA), IL-6 (A0286; ABclonal, Wuhan, China), α-Tub (AF7010, Affinity, Cincinnati, OH, USA), Whole Body Plethysmography (WBP) system (Buxco Research Systems, Wilmington, NC, USA), H&E dye (BA4025, Baso Diagnostics Inc., Zhuhai, China), Image-Pro Plus 6.0 software package (Media Cybernetics, Inc., Rockville, MD, USA), V.G. dye (BA4084, BaSO Diagnostics Inc., Zhuhai, China), goat anti-rabbit or goat anti-mouse secondary antibodies (074-1506/074-1806; Kirkegaard and Perry Laboratories, Gaithersburg, MD, USA), ECL prime western blotting detection reagent (ZD310A; ZomanBio, Beijing, China), and SPSS 20.0 software (IBM Corp., Armonk, NY, USA).
4.2. Establishment of the Mice Models
All of the study protocols were approved by the Committee on the Ethics of North China University of Science and Technology (LX2019033) and complied with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. A total of 120 C57BL/6 mice aged four weeks were purchased from Vital River Laboratory Animal Technology, China, and maintained at 12 h light/12 h dark period with free access to food and water.
The mice were divided into four groups as follows: control male (n = 30) and control female (n = 30) and male (n = 30) and female (n = 30) treatment groups. Both the control groups were subjected to the tracheal perfusion with 50 µL of 0.9% normal saline, whereas both the treatment groups were treated with tracheal perfusion with a 10 mg silica suspension (50 µL), using a small animal laryngoscope [7]. The mice were sacrificed at two, four, and six weeks, respectively. Left lobe tissues were dehydrated and embedded in paraffin, while the right lobe tissues were stored at −80 °C for the western blot.
4.3. Non-Invasive Measurement of the Pulmonary Function
The pulmonary functions were measured in the conscious mice with the FinePointe WBP system, as per the manufacturer’s instructions. The pulmonary function index values were automatically detected every 2 s. The measurement parameters were set as follows: 8 min for the duration of adaptation, 1 s for the nebulization, 5 min for the response, and 1 min for the recovery. The main indexes included the tidal volume (Tvb), minute volume (Mvb), peak expiratory flow (PEF), enhanced pause (Penh), pause (PAU), and expiratory flow 50 (EF50).
4.4. H&E Staining
Paraffin-embedded lung tissue sections were deparaffinized and rehydrated. The H&E dye were then added, dropwise, to observe the histopathological morphology. The area of the silicon nodules were counted using Image-Pro Plus 6.0 software package and then homogenized by the total area.
4.5. V.G. Staining
The de-waxed rehydrated lung tissue sections were treated with an equal proportion of hematoxylin A and hematoxylin B compared, and further, the V.G. dye was added. The area of collagen fiber stained by the V.G. dye was counted by Image-Pro Plus 6.0 software package and then homogenized by the total area.
4.6. Western Blot Assay
The proteins were separated using SDS-PAGE and further detected using the western blotting as described previously [56]. Briefly, the membranes were treated with primary antibodies of COL I, α-SMA, vimentin, TGF-β1, p-SMAD2/3, p21, p16, and α-Tub, respectively, (diluted at 1:1000). Further, they were incubated with goat anti-rabbit or goat anti-mouse secondary antibodies (diluted at 1:5000) in a blocking buffer. The immunoblots were visualized using the ECL prime western blotting detection reagent. The results were normalized against the corresponding control.
4.7. Statistical Analysis
The statistical analyses were performed using SPSS 20.0 software. Two-group comparisons were made using an unpaired Student’s t-test, and for multiple-group comparisons, a Shapiro—Wilk test was used to check the distribution of our data, and anova was used if the data were normally distributed, and the nonparametric tests were used if the data were not normally distributed. The statistical significance was achieved when p < 0.05 at a 95% confidence interval.
Author Contributions
Conceptualization, H.X., Z.W. and H.L.; Data curation, F.J., Y.L., X.W. and X.Y.; Formal analysis, Y.L. and T.L.; Investigation, Y.L. and T.L.; Methodology, F.J., X.W. and X.Y.; Project administration, F.J. and H.X.; Resources, Z.W. and H.L.; Software, Z.W.; Supervision, H.X. and H.L.; Writing—original draft, F.J. and Y.L.; Writing—review & editing, Z.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and ap-proved by the Committee on the Ethics of North China University of Science and Technology (protocol code LX2019033 and 2019-3-3 of approval).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the National Natural Science Foundation of China (82204006), the Natural Science Foundation of Hebei Province (H2020209073), the Science and Technology of Project of Hebei Education Department (ZD2022127).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li J., Yin P., Wang H., Wang L., You J., Liu J., Liu Y., Wang W., Zhang X., Niu P., et al. The burden of pneumoconiosis in China: An analysis from the Global Burden of Disease Study 2019. BMC Public Health. 2022;22:1114. doi: 10.1186/s12889-022-13541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Z., Zhao Y., Sun D. China’s occupational health challenges. Occup. Med. 2017;67:87–90. doi: 10.1093/occmed/kqw102. [DOI] [PubMed] [Google Scholar]
- 3.The Lancet Respiratory Medicine The world is failing on silicosis. Lancet Respir. Med. 2019;7:283. doi: 10.1016/S2213-2600(19)30078-5. [DOI] [PubMed] [Google Scholar]
- 4.Li T., Yang X.-Y., Xu D.-J., Gao Z.-Y., Gao Y.-B., Jin F.-Y., Li Y.-Q., Liu S.-P., Li S.-F., Gao X.-M., et al. OC-STAMP Overexpression Drives Lung Alveolar Epithelial Cell Type II Senescence in Silicosis. Oxid. Med. Cell. Longev. 2021;2021:4158495. doi: 10.1155/2021/4158495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin F., Geng F., Xu D., Li Y., Li T., Yang X., Liu S., Zhang H., Wei Z., Li S., et al. Ac-SDKP Attenuates Activation of Lung Macrophages and Bone Osteoclasts in Rats Exposed to Silica by Inhibition of TLR4 and RANKL Signaling Pathways. J. Inflamm. Res. 2021;14:1647–1660. doi: 10.2147/JIR.S306883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao N., Fan Y., Liu W., Yang H., Yang Y., Li Y., Jin F., Li T., Yang X., Gao X., et al. Oxamate Attenuates Glycolysis and ER Stress in Silicotic Mice. Int. J. Mol. Sci. 2022;23:3013. doi: 10.3390/ijms23063013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Cai W., Jin F., Wang X., Liu W., Li T., Yang X., Liu H., Xu H. Thalidomide Alleviates Pulmonary Fibrosis Induced by Silica in Mice by Inhibiting ER Stress and the TLR4-NF-κB Pathway. Int. J. Mol. Sci. 2022;23:5656. doi: 10.3390/ijms23105656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geng F., Xu M., Zhao L., Zhang H., Li J., Jin F., Li Y., Li T., Yang X., Li S., et al. Quercetin Alleviates Pulmonary Fibrosis in Mice Exposed to Silica by Inhibiting Macrophage Senescence. Front. Pharm. 2022;13:912029. doi: 10.3389/fphar.2022.912029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao X., Xu H., Xu D., Li S., Wei Z., Li S., Cai W., Mao N., Jin F., Li Y., et al. MiR-411-3p alleviates Silica-induced pulmonary fibrosis by regulating Smurf2/TGF-β signaling. Exp. Cell Res. 2020;388:111878. doi: 10.1016/j.yexcr.2020.111878. [DOI] [PubMed] [Google Scholar]
- 10.Laney A.S., Blackley D.J., Halldin C.N. Radiographic disease progression in contemporary US coal miners with progressive massive fibrosis. Occup. Environ. Med. 2017;74:517–520. doi: 10.1136/oemed-2016-104249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackley D.J., Reynolds L.E., Short C., Carson R., Storey E., Halldin C.N., Laney A.S. Progressive massive fibrosis in coal miners from 3 Clinics in Virginia. JAMA. 2018;319:500–501. doi: 10.1001/jama.2017.18444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maxfield R., Alo C., Reilly M.J., Rosenman K., Kalinowski D., Stanbury M., Valiante D.J., Jones B., Randolph S., Socie E., et al. Surveillance for silicosis, 1993–Illinois, Michigan, New Jersey, North Carolina, Ohio, Texas, and Wisconsin. MMWR CDC Surveill. Summ. 1997;46:13–28. [PubMed] [Google Scholar]
- 13.Pinkerton K.E., Harbaugh M., Han M.K., Saux C.J.L., Winkle L.S.V., Martin W.J., Kosgei R.J., Carter E.J., Sitkin N., Smiley-Jewell S.M. Women and Lung Disease. Sex Differences and Global Health Disparities. Am. J. Respir. Crit. Care Med. 2015;192:11–16. doi: 10.1164/rccm.201409-1740PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein S.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 15.Ballesteros B.L.-M., López-Herranz M., Lopez-de-Andrés A., Hernandez-Barrera V., Jiménez-García R., Carabantes-Alarcon D., Jiménez-Trujillo I. Sex Differences in the Incidence and Outcomes of Patients Hospitalized by Idiopathic Pulmonary Fibrosis (IPF) in Spain from 2016 to 2019. J. Clin. Med. 2021;10:3474. doi: 10.3390/jcm10163474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solopov P., Biancatelli R.M.L.C., Dimitropoulou C. Sex-Related Differences in Murine Models of Chemically Induced Pulmonary Fibrosis. Int. J. Mol. Sci. 2021;22:5909. doi: 10.3390/ijms22115909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You D.J., Lee H.Y., Taylor-Just A.J., Linder K.E. Sex differences in the acute and subchronic lung inflammatory responses of mice to nickel nanoparticles. Nanotoxicology. 2020;14:1058–1081. doi: 10.1080/17435390.2020.1808105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y., Jin F., Li T., Yang X., Cai W., Li S., Gao X., Mao N., Liu H., Xu H. Minute Cellular Nodules as Early Lesions in Rats with Silica Exposure via Inhalation. Vet. Sci. 2022;9:251. doi: 10.3390/vetsci9060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H., Yang F., Sun Y., Yuan Y., Cheng H., Wei Z., Li S., Cheng T., Brann D. A new antifibrotic target of Ac-SDKP: Inhibition of myofibroblast differentiation in rat lung with silicosis. PLoS ONE. 2012;7:e40301. doi: 10.1371/journal.pone.0040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu H., Yu Y., Huang H., Hu Y., Fu S., Wang Z., Shi M., Zhao X., Yuan J., Li J., et al. Progressive Pulmonary Fibrosis Is Caused by Elevated Mechanical Tension on Alveolar Stem Cells. Cell. 2020;180:107–121.e17. doi: 10.1016/j.cell.2019.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Poinen-Rughooputh S., Rughooputh M.S., Guo Y., Lai H., Sun W. Sex-Related Differences in the Risk of Silicosis Among Chinese Pottery Workers: A Cohort Study. J. Occup. Environ. Med. 2021;63:74–79. doi: 10.1097/JOM.0000000000002068. [DOI] [PubMed] [Google Scholar]
- 22.Hoy R.F. Silica-related diseases in the modern world. Allergy. 2020;75:2805–2817. doi: 10.1111/all.14202. [DOI] [PubMed] [Google Scholar]
- 23.Bayram H. Killer jeans and silicosis. Am. J. Respir. Crit. Care Med. 2011;184:1322–1324. doi: 10.1164/rccm.201108-1440ED. [DOI] [PubMed] [Google Scholar]
- 24.Conti S., Harari S., Caminati A., Zanobetti A., Schwartz J.D., Bertazzi P.A., Cesana G. The association between air pollution and the incidence of idiopathic pulmonary fibrosis in Northern Italy. Eur. Respir. J. 2018;51:1700397. doi: 10.1183/13993003.00397-2017. [DOI] [PubMed] [Google Scholar]
- 25.Angelidis I., Simon L.M., Fernandez I.E., Strunz M., Mayr C.H., Greiffo F.R., Tsitsiridis G., Ansari M., Graf E., Strom T., et al. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat. Commun. 2019;10:963. doi: 10.1038/s41467-019-08831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deutsch G.H., Young L.R., Deterding R.R., Fan L.L., Dell S.D., Bean J.A., Brody A.S., Nogee L.M., Trapnell B.C., Langston C., et al. Diffuse lung disease in young children: Application of a novel classification scheme. Am. J. Respir. Crit. Care Med. 2007;176:1120–1128. doi: 10.1164/rccm.200703-393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liptzin D.R., Landau L.I. Sex and the lung: Observations, hypotheses, and future directions. Pediatr. Pulmonol. 2015;50:1159–1169. doi: 10.1002/ppul.23178. [DOI] [PubMed] [Google Scholar]
- 28.Gharaee-Kermani M., Hatano K., Nozaki Y., Phan S.H. Gender-Based Differences in Bleomycin-Induced Pulmonary Fibrosis. Am. J. Pathol. 2005;166:1593–1606. doi: 10.1016/S0002-9440(10)62470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walters D.M. Mouse models of bleomycin-induced pulmonary fibrosis. Curr. Protoc. Pharmacol. 2008;40:5-46. doi: 10.1002/0471141755.ph0546s40. [DOI] [PubMed] [Google Scholar]
- 30.Haston C.K., Amos C.I., King T.M. Inheritance of susceptibility to bleomycin-induced pulmonary fibrosis in the mouse. Cancer Res. 1996;56:2596–2601. [PubMed] [Google Scholar]
- 31.Leung C.C., Yu I.T.S., Chen W. Silicosis. Lancet. 2012;379:2008–2018. doi: 10.1016/S0140-6736(12)60235-9. [DOI] [PubMed] [Google Scholar]
- 32.Lai Y.-T., Huang Y.-S. Modification and Application of a Commercial Whole-body Plethysmograph to Monitor Respiratory Abnormalities in Neonatal Mice. Bio-Protoc. 2017;7:e2343. doi: 10.21769/BioProtoc.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shumin L., Min Z., Peng W., Fan H., Chen W., Yue Z., Zhenyang G., Xinyu Y., Xiaoxiao H., Ying S., et al. Determination of noninvasive pulmonary function in mice and its significance. Acta Lab. Anim. Sci. Sin. 2018;26:548–553. [Google Scholar]
- 34.Pollard K.M. Perspective: The lung, particles, fibers, nanomaterials, and autoimmunity. Front. Immunol. 2020;11:587136. doi: 10.3389/fimmu.2020.587136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adamcakova J., Mokra D. New Insights into Pathomechanisms and Treatment Possibilities for Lung Silicosis. Int. J. Mol. Sci. 2021;22:4162. doi: 10.3390/ijms22084162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buskiewicz I.A., Huber S.A., Fairweather D. Sex Differences in Physiology. Academic Press; Amsterdam, The Netherlands: 2016. Sex hormone receptor expression in the immune system; pp. 45–60. [Google Scholar]
- 37.Di Florio D.N., Sin J., Coronado M.J., Atwal P.S., Fairweather D. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. 2020;31:101482. doi: 10.1016/j.redox.2020.101482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He R., Yuan X., Lv X., Liu Q., Tao L., Meng J. Caveolin-1 negatively regulates inflammation and fibrosis in silicosis. J. Cell Mol. Med. 2022;26:99–107. doi: 10.1111/jcmm.17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du S., Li C., Lu Y., Lei X., Zhang Y., Li S., Liu F., Chen Y., Weng D., Chen J. Dioscin Alleviates Crystalline Silica-Induced Pulmonary Inflammation and Fibrosis through Promoting Alveolar Macrophage Autophagy. Theranostics. 2019;9:1878–1892. doi: 10.7150/thno.29682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayeux J.M., Escalante G.M., Christy J.M., Pawar R.D., Kono D.H., Pollard K.M. Silicosis and Silica-Induced Autoimmunity in the Diversity Outbred Mouse. Front. Immunol. 2018;9:874. doi: 10.3389/fimmu.2018.00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Budi E.H., Schaub J.R., Decaris M., Turner S. TGF-β as a driver of fibrosis: Physiological roles and therapeutic opportunities. J. Pathol. 2021;254:358–373. doi: 10.1002/path.5680. [DOI] [PubMed] [Google Scholar]
- 42.Li S., Wei Z., Li G., Zhang Q., Niu S., Xu D., Mao N., Chen S., Gao X., Cai W., et al. Silica Perturbs Primary Cilia and Causes Myofibroblast Differentiation during Silicosis by Reduction of the KIF3A-Repressor GLI3 Complex. Theranostics. 2020;10:1719–1732. doi: 10.7150/thno.37049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao X., Xu D., Li S., Wei Z., Li S., Cai W., Mao N., Jin F., Li Y., Yi X., et al. Pulmonary Silicosis Alters MicroRNA Expression in Rat Lung and miR-411-3p Exerts Anti-fibrotic Effects by Inhibiting MRTF-A/SRF Signaling. Mol. Ther. Nucleic Acids. 2020;20:851–865. doi: 10.1016/j.omtn.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai W., Xu H., Zhang B., Gao X., Li S., Wei Z., Li S., Mao N., Jin F., Li Y., et al. Biomedecine, p.; pharmacotherapie, Differential expression of lncRNAs during silicosis and the role of LOC103691771 in myofibroblast differentiation induced by TGF-β1. Biomed. Pharmacother. 2020;125:109980. doi: 10.1016/j.biopha.2020.109980. [DOI] [PubMed] [Google Scholar]
- 45.Shah T.A. Unanswered Questions Regarding Sex and BMP/TGF-β Signaling. J. Dev. Biol. 2018;6:14. doi: 10.3390/jdb6020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito I., Hanyu A., Wayama M., Goto N., Katsuno Y., Kawasaki S., Nakajima Y., Kajiro M., Komatsu Y., Fujimura A., et al. Estrogen inhibits transforming growth factor beta signaling by promoting Smad2/3 degradation. J. Biol. Chem. 2010;285:14747–14755. doi: 10.1074/jbc.M109.093039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziller N., Kotolloshi R., Esmaeili M., Liebisch M., Mrowka R., Baniahmad A., Liehr T., Wolf G. Sex Differences in Diabetes- and TGF-β1-Induced Renal Damage. Cells. 2020;9:2236. doi: 10.3390/cells9102236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diamond-Stanic M.K., You Y.H. Sugar, sex, and TGF-β in diabetic nephropathy. Semin. Nephrol. 2012;32:261–268. doi: 10.1016/j.semnephrol.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rocca W.A., Rocca L.G., Smith C.Y., Grossardt B.R., Faubion S.S., Shuster L.T., Kirkland J.L., LeBrasseur N.K., Schafer M.J., Mielke M.M., et al. Loss of Ovarian Hormones and Accelerated Somatic and Mental Aging. Physiology. 2018;33:374–383. doi: 10.1152/physiol.00024.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farr J.N., Rowsey J.L., Eckhardt B.A., Thicke B.S., Fraser D.G., Tchkonia T., Kirkland J.L., Monroe D.G. Independent Roles of Estrogen Deficiency and Cellular Senescence in the Pathogenesis of Osteoporosis: Evidence in Young Adult Mice and Older Humans. J. Bone Miner. Res. 2019;34:1407–1418. doi: 10.1002/jbmr.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei Y., Fu J., Wu W., Ma P., Ren L., Wu J. Estrogen prevents cellular senescence and bone loss through Usp10-dependent p53 degradation in osteocytes and osteoblasts: The role of estrogen in bone cell senescence. Cell Tissue Res. 2021;386:297–308. doi: 10.1007/s00441-021-03496-7. [DOI] [PubMed] [Google Scholar]
- 52.Sharpless N.E. Forging a signature of in vivo senescence. Nat. Rev. Cancer. 2015;15:397–408. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- 53.Kerget B., Araz O., Ucar E.Y., Karaman A., Calik M., Alper F. Female workers’ silicosis diagnosis delayed due to gender bias. Occup. Med. 2019;69:219–222. doi: 10.1093/occmed/kqz019. [DOI] [PubMed] [Google Scholar]
- 54.Forastiere F., Goldsmith D.F., Sperati A., Rapiti E., Miceli M., Cavariani F., Perucci C.A. Silicosis and lung function decrements among female ceramic workers in Italy. Am. J. Epidemiol. 2002;156:851–856. doi: 10.1093/aje/kwf120. [DOI] [PubMed] [Google Scholar]
- 55.Ndlovu N., Richards G., Vorajee N., Murray J. Silicosis and pulmonary tuberculosis in deceased female South African miners. Occup. Med. 2019;69:272–278. doi: 10.1093/occmed/kqz067. [DOI] [PubMed] [Google Scholar]
- 56.Shifeng L., Hong X., Xue Y., Siyu N., Qiaodan Z., Dingjie X., Lijuan Z., Zhongqiu W., Xuemin G., Wenchen C., et al. Ac-SDKP increases α-TAT 1 and promotes the apoptosis in lung fibroblasts and epithelial cells double-stimulated with TGF-β1 and silica. Toxicol. Appl. Pharmacol. 2019;369:17–29. doi: 10.1016/j.taap.2019.02.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.