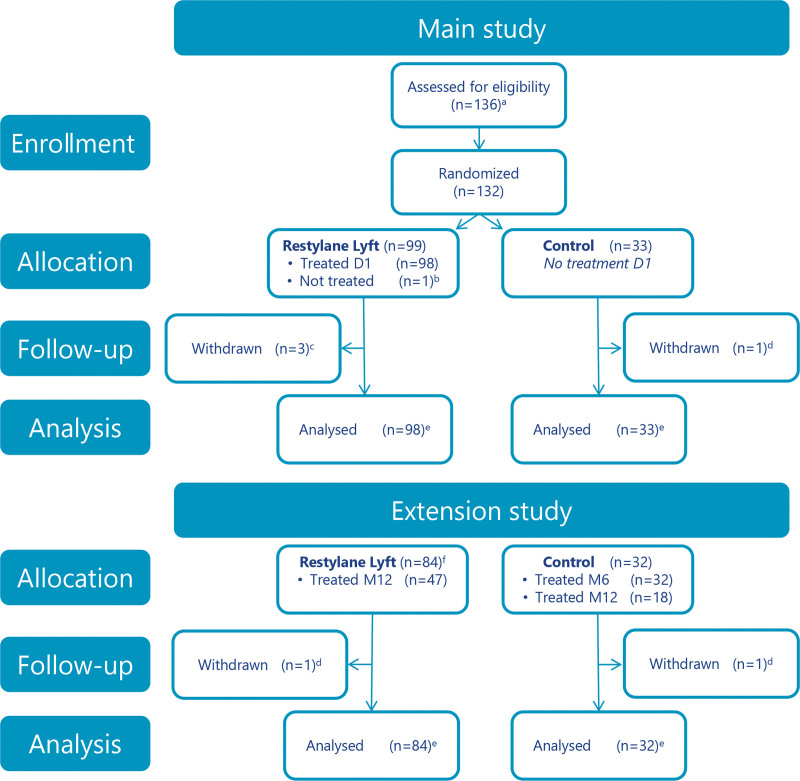
Fig. 2.
Subject disposition. aFour subjects were not randomized (did not meet eligibility criteria). bWithdrew consent. cLost to follow-up (n = 2); withdrew consent (n = 1). dLost to follow-up. eFull analysis and safety analysis sets. fSubjects consenting to participate in the extension study. Twelve subjects did not consent to participate in the extension study; of these, eight subjects received optional re-treatment at month 6. D1, day 1; M6, month 6; M12, month 12.