Table 7.
Compilation of favorable substitutions and SAR for triazole and tetrazole molecules with a traditional azole pharmacophore.
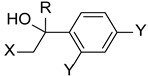
| |||
|---|---|---|---|
| X | Y | R | SAR |
Triazole 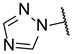
|
F |
|
R1 = Halogens, methyl, cyanide, and nitro groups led to promising antifungal potential against Candida albicans, Candida parapsilosis, Cryptococcus neoformans, and Nannizzia gypsea |
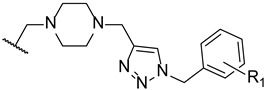
| F or Cl |
|
R1 = Di-chloro-phenyl, paired with Y = Cl, led to the highest antifungal potential of the tested series Y = Cl increased antifungal activity against C. albicans, C. parapsilosis, C. neoformans, Epidermophyton floccosum and Trichophyton mentagrophytes |
|
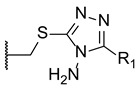
| F |
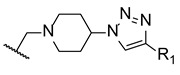
|
R1 = butyrate and butyric acid increased antifungal potential against all tested strains; 4-acetyl or 4-trifluoromethoxy-phenyl groups increased activity for C. albicans; 2-methyl-phenyl group increased activity against C. parapsilosis and Candida glabrata | |
| F or Cl |
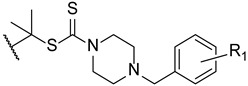
|
R1 = 4-Chloro showed high antifungal activity against C. albicans, C. glabrata, C. parapsilosis, Candida krusei and Candida tropicalis. Y = F analogs presented higher antifungal potency |
|
| F |
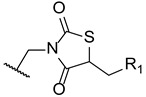
|
R1 = Di-chloro-phenyl, furan ring presented promising results against C. albicans | |
| F | 1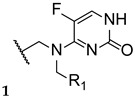
|
1: R1 = alkyl chains were not beneficial for antifungal activity 2: R1–3 = one fluor substituent; two chlorine substituents: increased antifungal activity against C. albicans |
|
2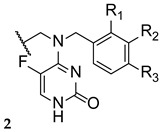
| |||
| F | 1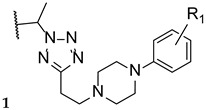
|
1 and 2: positional isomers R1 = trifluoromethyl led to high antifungal activity against C. albicans, C. tropicalis, C. parapsilosis, C. krusei, C. glabrata, C. neoformans, Aspergillus fumigatus, and Aspergillus niger |
|
2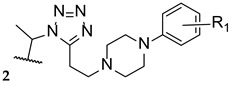
| |||
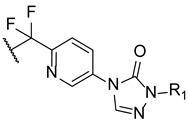
Tetrazole 
|
F |
|
R1 = Alicyclic side chains up to six-members enhance the antifungal potency against C. albicans, C. parapsilosis, C. glabrata, C. neoformans, and A. fumigatus |
