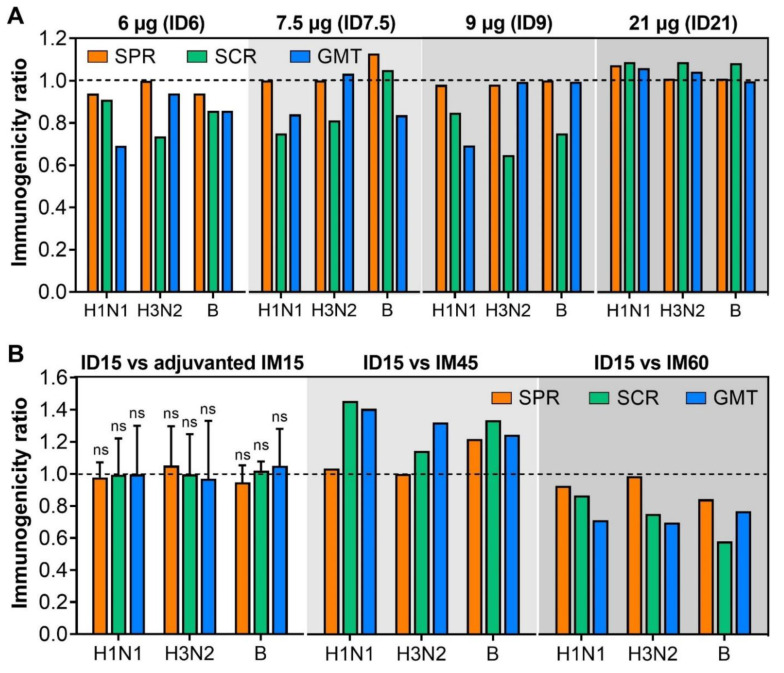
Figure 2.
Comparison of the immunogenicity induced by varying amounts of hemagglutinin antigen in ID vaccines (A) and by standard-dose ID vaccines and MF59-adjuvanted IM15 and high-dose IM vaccines (B) in the elderly. (A) Comparison of immunogenicity induced by various dosages, including 6 µg (ID6), 7.5 µg (ID7.5), 9 µg (ID7.5), 21 µg (ID21) and standard dosage (15 µg) of influenza vaccines injected intradermally. The ratios of SPR, SCR, and GMT were calculated by dividing the mean SPR, SCR, and GMT of ID vaccination of various dosages by those of standard-dose ID15. A ratio of <1 (dash line) implies a lower immunogenicity of varying dosages of ID vaccination than ID15. (B) Comparison of immunogenicity of standard-dose ID15 and MF59-adjuvanted IM15, high dose (45 µg or IM45, 60 µg or IM60) of IM vaccines. The ratios of SPR, SCR, and GMT were calculated by dividing the mean SPR, SCR, and GMT of ID15 by those of adjuvanted IM15, IM45, and IM60. A ratio of >1 (dash line) implies a higher immunogenicity of ID15 over adjuvanted ID15, IM45, and IM60. One sample t-test was used to examine the significant difference in the immunogenicity ratios against hypothesized ratio of 1. “ns” = non-significant. Due to limited studies comparing the immunogenicity of ID15 and ID vaccines at varying amounts of hemagglutinin antigen, ID15 and high-dose IM vaccines, a statistical test was not performed.