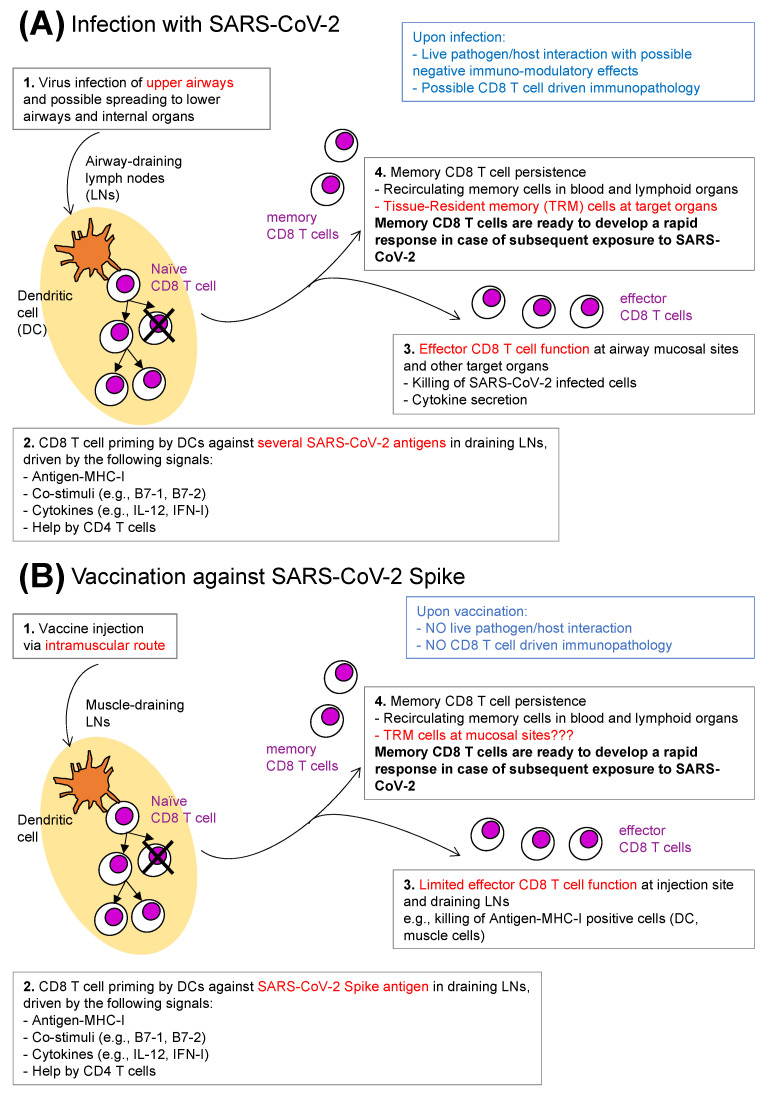
Figure 1.
Anti-SARS-CoV-2 CD8 T cells: natural infection versus vaccination. (A) Infection with SARS-CoV-2. SARS-CoV-2 infection usually starts in the upper respiratory airways, with possible subsequent virus spreading to other organs (1). Priming of naïve CD8 T cells occurs in airway-draining lymph nodes (LNs), wherein mature dendritic cells (DCs) present epitopes from a series of SARS-CoV-2 antigens in the context of MHC-I molecules, together with ligands for costimulatory receptors, and in the presence of additional signals provided by helper CD4 T cells and cytokines (2). Primed CD8 T cells proliferate and differentiate, generating a progeny of CD8 T cells that migrate out of LNs and reach distant sites all over the body. Upon antigen–MHC-I recognition on the surface of SARS-CoV-2-infected cells in so-called target organs, short-lived effector CD8 T cells exert their function, e.g., cytotoxicity and cytokine secretion (3). Memory CD8 T cells recirculate in blood and lymphoid organs and are found as differentiated tissue-resident memory CD8 T cells in target organs (4). Possible limitations of the CD8 T cell-mediated protective function against SARS-CoV-2 infection are listed in blue. (B) Vaccination against SARS-CoV-2 spike. A typical CD8 T cell response elicited by a vaccine encoding for the spike antigen of SARS-CoV-2 (e.g., adenovirus- or mRNA-based vaccine) is schematically depicted. After vaccine injection via the intramuscular route (1), the few naïve CD8 T cells that are specific for spike epitopes clonally expand and differentiate in muscle-draining LNs, wherein CD8 T cell priming occurs (2). Effector CD8 T cell function is expected to be very limited after a single vaccine injection, and more pronounced after repeated vaccine doses; it mostly consists of killing of spike-expressing muscle cells and DCs, and of DCs that expose spike-derived peptides in the context of MHC-I after taking up debris from damaged spike-expressing cells (3). Anti-spike vaccines elicit memory CD8 T cells recirculating in blood and lymphoid organs, and possibly tissue-resident memory CD8 T cells at mucosal sites (4). ((A) vs. (B)). Memory CD8 T cells which persist either post-infection (A) or post-vaccination (B) develop a rapid response in case of subsequent encounter with SARS-CoV-2, contributing to protection (box 4 in (A,B), in bold). The main differences between A and B are highlighted in red (boxes 1–4 in (A,B)). Other differences remain to be determined, e.g., in respect to the quality/quantity of the priming signals listed in box 2 in A and B, to the durability of memory CD8 T cell response, etc. (not depicted). The limitations of CD8 T cell protective function listed in (A) are not applicable in (B) (in blue). See text for more details.