Abstract
Despite the health benefits associated with the ingestion of the bioactive compounds in cocoa, the high concentrations of polyphenols and methylxanthines in the raw cocoa beans negatively influence the taste, confer the astringency and bitterness, and affect the stability and digestibility of the cocoa products. It is, therefore, necessary to process cocoa beans to develop the characteristic color, taste, and flavor, and reduce the astringency and bitterness, which are desirable in cocoa products. Processing, however, affects the composition and quantities of the bioactive compounds, resulting in the modification of the health-promoting properties of cocoa beans and chocolate. In this advanced review, we sought to better understand the effect of cocoa’s transformational process into chocolate on polyphenols and methylxanthine and the mechanism of action of the original flavanols and methylxanthines. More data on the cocoa processing effect on cocoa bioactives are still needed for better understanding the effect of each processing step on the final polyphenolic and methylxanthine composition of chocolate and other cocoa products. Regarding the mechanisms of action, theobromine acts through the modulation of the fatty acid metabolism, mitochondrial function, and energy metabolism pathways, while flavanols mainly act though the protein kinases and antioxidant pathways. Both flavanols and theobromine seem to be involved in the nitric oxide and neurotrophin regulation.
Keywords: cocoa processing, chocolate, flavanol, polyphenol, methylxanthine, molecular mechanism
1. Introduction
Cocoa (Theobroma cacao L.) belongs to the family Malvaceae and the genus Theobroma. The cocoa tree produces fruits, referred to as pods, along the trunk and branches. The pods are oval and contain the seeds (about 30–40 seeds), commonly known as cocoa beans, which are embedded in a sweet mucilaginous pulp. The pulp is rich in fermentable sugars and high in acidity (pH 3.0–3.5). Chocolate is the most popular cocoa-derived product, highly valued by consumers around the world [1,2,3]. Its consumption has received increased global attention in recent years due to the biologically active components in cocoa beans [1,2,3,4]. However, some adverse effects of chocolate consumption have also been reported, as the final chocolate might be rich in sugar and fat [5]. This limits the use of chocolate as a functional food product and opens a search for more “natural” and unprocessed forms of cocoa. The components of the cocoa bean quality currently used on the international cocoa market include the bean size and mass, moisture content, flavor characteristics, low debris and bean defect levels, fat quality, and content [6,7]. There is a growing global trend in the search for functional attributes in food as quality indicators. In the case of cocoa beans, the final qualitative and quantitative content of the polyphenols and methylxanthines might be considered as part of the quality indicators affecting the final price of the beans.
The present revision will focus on two different types of phytochemicals in cocoa, the main cocoa polyphenols, flavanols, and the foremost cocoa methylxanthine, theobromine, because this compound is as abundant in cocoa as the total of the flavonoids and is currently the subject of an increasing number of studies [8,9,10,11]. Cocoa flavanols and methylxanthines control the molecular pathways that regulate the cell signaling and function in most of the tissues so far investigated. The main pathways studied include the mitogen-activated protein kinase (MAPK) pathways and phosphoinositide-3-kinase-protein kinase B/protein kinase B (PI3K/Akt), which are activated by several ubiquitous growth factors, such as the insulin-like growth factor-1 (IGF-1) and fibroblast growth factor (FGF), among others, and neuro-specific factors, such as the brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) [12,13]. Additionally, to the canonical signaling pathways, the systemic factors and molecular/biochemical mechanisms, such as nitric oxide (NO), toll-like receptors, apoptosis, and antioxidant and anti-inflammatory responses, are also specifically involved in the biological effects of cocoa phytochemicals on the tissue and cell function, and these will be discussed in this review. It is worth mentioning that most, if not all, of these pathways are interconnected, and it results in being very difficult most times to unravel and delineate the specific effects. Moreover, several polyphenols included in a diet may affect more than one biomarker or pathway, which may confound conclusions about the specificity of action [12,13,14]. Raw cocoa beans are bitter and astringent and need to be processed to reduce the bitterness and astringency and develop the desired flavors. The bitterness and astringency of raw cocoa beans are due to the phenolics and methylxanthines in the beans [3]. During the cocoa bean processing, a wide range of chemical reactions, including the aldol condensation, polymerization, cyclization, Maillard reaction, and Strecker degradation, which enhance the flavor, color, and shelf stability of cocoa products, have been reported [15,16,17,18,19,20,21]. According to some authors, the polyphenols undergo a biochemical modification through the polymerization and complexation with other compounds such as proteins [6,16], while theobromine and caffeine mainly diffuse into the shells of the beans [22,23]. These result in a decrease in the concentration of the phenolic and methylxanthine compounds and contribute to the reduction in the bitterness and astringency of the beans [22,23]. However, there is no general agreement regarding the negative effect of food processing on the content of the bioactive compounds in processed cocoa products. In fact, as occurs with other processed foods, some authors have shown that processing, far from reducing the biological activity of foods of plant origin, could enhance their bioavailability and, beyond that, their bioactivity [24,25]. Moreover, during processing, notably the fermentation and drying processes, flavor precursors (free amino acids and reducing sugars) are formed, and these combine during the roasting process through a complex series of thermal reactions, mainly the Maillard reaction and Strecker degradation, and are converted into desirable aroma volatiles, such as pyrazines, aldehydes, ketones, furans, and pyrroles [18,19,20,21]. Nevertheless, cocoa processing clearly affects the flavanols and methylxanthine content of the final product and therefore will affect its potential biological activity and the molecular mechanisms that mediate it.
The purpose of this review is to provide a better understanding of the various processing steps of cocoa beans, from harvesting to chocolate production, emphasizing the changes in the main bioactive compounds in cocoa: the polyphenols (mainly flavanols) and methylxanthines (mainly theobromine) during processing that will affect the mechanism of action implicated in their biological activity. The research gaps are also identified to aid in the planning of future studies. Because this review aims to be an update of the information regarding the flavanols and methylxanthines of cocoa, most of the results reported up to 2020 will be briefly summarized and largely referred to comprehensive reviews; henceforth, we will mainly focus on recent data from the last couple of years, in which, despite the pandemic, research on this topic has been rather productive.
2. Cocoa Bean Processing
The processing of cocoa beans can be categorized into primary and industrial processing. The primary processing of cocoa entails all the processes cocoa pods go through, from harvesting to obtaining the final dried beans (Figure 1). They play a significant role in the development of the final color, flavor profile, and composition of the bioactive compounds of the dried beans as well as the shelf stability of the beans during transportation and storage [26,27]. These include harvesting, pod storage (as a means of pulp pre-conditioning), pod breaking, fermentation, and drying. Although cocoa bean quality is greatly influenced by the genetic makeup and origin of the cocoa, inadequate or poor primary processing could result in beans with a poor quality and shelf stability.
Figure 1.
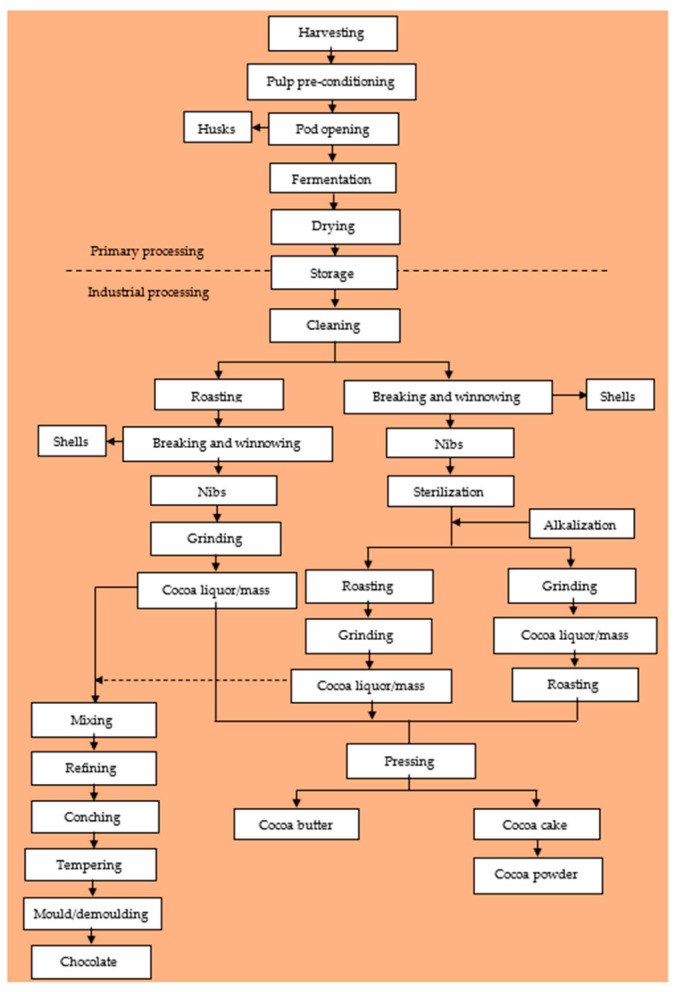
Schematic diagram of cocoa bean processing and chocolate production.
The industrial processing of cocoa can also be sub-classified into secondary and tertiary processing. The secondary processing involves all the processes the dried fermented beans are taken through to obtain semi-finished products, such as cocoa liquor (cocoa mass), cocoa butter, cocoa powder, etc. The secondary processing includes cleaning, breaking and winnowing, sterilization, alkalization, roasting, nib grinding, and liquor processing (Figure 1). The tertiary processing involves the use of the semi-finished cocoa products and other ingredients to produce chocolates, cocoa/chocolate beverages, and other confectionery products.
2.1. Primary Processing of Cocoa Beans
2.1.1. Harvesting
The harvesting of ripe, matured cocoa pods initiates the primary processing of cocoa. For optimal processing and the production of high-quality beans for cocoa-based products, only matured, ripe, or at least semi-ripe, and disease-free pods are harvested. The Studies have reported on the direct influence of harvesting cocoa pods at an appropriate maturity stage on the flavor characteristics of the cocoa beans [28]. The cocoa beans obtained from unripe pods have been found to have low sugar contents and do not ferment properly, while over-ripe ones, on the other hand, are easy to germinate and be infected by microorganisms [29,30]. In terms of the bioactive compounds in cocoa beans, studies have shown that cocoa beans obtained from immature or over-ripe fruits contain fewer bioactive compounds than beans from fully mature fruits [30,31]. A recent study by Dang and Nguyen [32] showed that the maturity at harvesting and the fermentation conditions significantly increased the flavanol and methylxanthine contents, together with the antioxidant capacity of the cocoa beans (Table 1). Earlier studies by Zheng et al. [33] and Pereira-Caro et al. [30] found the theobromine and caffeine contents appeared at maturity when the cocoa beans started to develop their seed coats, which continued to increase until the cocoa fruits were fully ripe. Zheng et al. [33] also explained that the increase in the alkaloid compounds could be due to their transport from the pericarp to the cocoa bean during fruit maturation, resulting in their increased concentration in the beans of matured fruits. Pereira-Caro et al. [30] noted that because the alkaloid compounds were located in the same storage cell as the phenolics, they could develop together with the phenolics during maturation.
Table 1.
Processing effects on polyphenolic and methylxanthines composition in cocoa beans and chocolate.
| Stage | Process | Changes Occurring | Antioxidant Capacity | References |
|---|---|---|---|---|
| Primary processing | Harvesting | Increased proanthocyanidins, CF, and TBR with increasing pod maturation and ripening at harvest. | Increase in the antioxidant capacity with an increase in pod maturation and ripening. | [30,32] |
| Pod storage | Reduction in TP, EC, C, TBR, and CF with increasing duration of pod storage. | Decrease in the antioxidant capacity with increasing duration of pod storage during fermentation. | [18,34,35,36] | |
| Fermentation | Decrease in the concentrations of EC, C, anthocyanins, phenolic acids, and TP with increasing duration of fermentation. Moreover, a decrease in the TBR and CF concentrations. | Decrease in the antioxidant capacity of cocoa beans with increasing duration of fermentation. | [18,22,23,37,38,39,40,41,42] | |
| Drying | Degradation of TP, EC, C, and anthocyanins. Decrease in the alkaloids (TBR and CF) content. | Reduction in the antioxidant capacity during the drying of cocoa beans. | [39,40,42,43,44,45] | |
| Industrial processing | Roasting | Reduction in the amounts of TP, EC, procyanidin B2 and C1, anthocyanins, quercetin glycosides, TBR, and CF. An increase in C due to epimerization of EC. | A general reduction in the antioxidant capacity during cocoa bean roasting. | [22,23,30,40,41,42,45,46,47,48,49,50,51,52] |
| Alkalization | Reduction in TP, EC, C, procyanidin B2 and C1, quercetin, quercetin-3-glucuronide, quercetin-3-arabinoside, isoquercetin, TBR, and CF. | Antioxidant activity is reduced. | [20,21,40,42,47,53,54] | |
| Conching | Decrease in TP during conching. No significant change in the polyphenol content during conching. |
Decrease in the antioxidant capacity. | [52,55,56,57,58,59,60] |
NB: TP—Total polyphenols; EC—Epicatechin; C—Catechin; TBR—Theobromine; CF—Caffeine.
2.1.2. Pod Storage
The cocoa pulp is the substrate sequentially metabolized by microorganisms during the fermentation process. The properties of the substrate determine the type and quantities of the microbial development and metabolism during the fermentation, hence changes in the pulp may affect the production of alcohols by the yeasts and the subsequent production of acids by the lactic acid and acetic acid bacteria [6]. Pod storage (PS) is storing harvested cocoa pods for a period before opening the pods and fermenting the beans. The harvested cocoa pods are living tissues and undergo metabolic activity, such as respiration and transpiration, using the sugars in the pulp. This activity leads to decreased sugar content in the pulp for yeasts to utilize during the start of the microbial phase of fermentation, resulting in reduced alcohol production by the yeasts. The reduced alcohol production in turn results in a reduction in the acetic acid production by the acetic acid bacteria, which will diffuse into the beans to initiate the biochemical reactions. The post-harvest storage of cocoa pods has been reported to reduce the nib acidification during the subsequent fermentation, result in a reduction in acid notes, and increase the cocoa flavors in the resulting cocoa beans [26]. The pod storage of cocoa has also been found to cause a significant reduction in the polyphenolic and methylxanthine content of cocoa beans after the fermentation and drying (Table 1), thereby reducing the astringency and bitterness in the cocoa and cocoa products.
2.1.3. Pod Breaking
In general, the pod breaking involves opening the harvested pods to extract the wet beans for fermentation, and it is important not to damage the beans in this process. Cutting the beans will allow insects or molds to enter the bean.
2.1.4. Cocoa Bean Fermentation
Cocoa bean fermentation is crucial in making cocoa beans more valuable and stable. It also influences the marketability and acceptability of the beans. It is conducted for the development of the characteristic brown cocoa color and modification of the polyphenolic and pH, leading to a reduction in the astringency and bitterness of the final dried beans. The fermentation also generates the flavor precursors, namely the free amino acids and peptides, from the enzymatic degradation of the cocoa proteins, reducing sugars from the enzymatic degradation of the sucrose [27] from which the typical cocoa aroma is generated during the subsequent roasting process.
During the fermentation, the beans are removed from the pods and subjected to various activities of various microorganisms, which are prevalent within the surrounding environment. The changes in the cocoa beans produced by the fermentation occur in two stages: the microbial fermentation, which takes place in the pulp, and biochemical changes, which occur in the beans following the death of the embryo. Following the death of the embryo and the breakdown of the cell walls in the bean, endogenous seed enzymes (e.g., proteases, polyphenol oxidases, glycosidase, and invertase) and substrates (e.g., polyphenols, proteins, and sugars) interact and react in a specific manner [18,61].
The bioactive compounds in the beans are subjected to a biochemical modification through the polymerization and complexation with protein, hence decreasing the solubility and astringency [6]. Polyphenols generally decrease as the fermentation degree increases (Table 1). The activity of the polyphenol oxidases (PPOs) during the fermentation is associated with the reduction in the polyphenols [18]. Studies have also shown that the loss of polyphenols during fermentation is not only due to the oxidation process but also caused by the diffusion of the polyphenols into fermentation sweating [62]. The methylxanthine contents of cocoa beans have also been found to diffuse into the shells of the beans during fermentation, thus causing a reduction in the concentration and contributing to the reduction in the bitterness of the beans [22,23]. The purple-colored anthocyanins located in the specialized vacuoles within the cotyledon are hydrolyzed by glycosidases to anthocyanidins [63]. Thompson et al. [64] observed that the enzyme cleaves the sugar moieties galactose and arabinose attached to the anthocyanins. This results in the bleaching of the purple color of the beans to brown as well as the release of reducing sugars that can participate as precursors to reactions during the roasting. In general, different cocoa types require different degrees of fermentation, but usually, it takes from 4 to 7 days.
2.1.5. Drying of Fermented Cocoa Beans
The moisture content of the cocoa beans after fermentation is about 60%, and this needs to be reduced to around 7% by drying [65,66] to prevent a mold infestation and the subsequent formation of Ochratoxins during storage. Cocoa bean drying is, however, more than just reducing the moisture content of the beans as it also allows some of the chemical changes, which occurred during the fermentation stage, to continue and improve the bean color and flavor development [42,67]. During the drying of fermented cocoa beans, the active polyphenolic oxidase catalyzes the polyphenols oxidation reactions into quinones, which are subjected to further condensation with free amine and sulfhydryl groups, which leads to the synthesis of brown polymers, development of new flavor components, and loss of membrane integrity [6]. This helps to further reduce the bitterness and astringency and develop the chocolate brown color of well-fermented cocoa beans. The timing of the drying process is crucial as the rapid drying of the beans results in case hardening which prevents the outward migration of acetic acid from the beans, thus leading to a build-up of acidity in the beans [68,69]. On the other hand, if the drying is too slow, molds and off flavors can develop [68,70].
2.2. Industrial Processing of Cocoa Beans
2.2.1. Cleaning
Cocoa beans for processing are first evaluated for quality, which is important to the final product quality. The beans are then subjected to a cleaning process which involves the removal of all the extraneous materials from the cocoa beans. The process involves a sieving operation to remove all the extraneous materials, such as stones, strings, coins, wood pieces, soil particles, nails, etc. Cleaning before processing is essential to ensure the production of quality products devoid of extraneous materials and to also reduce the wear on machinery, as pieces of metal or stone can cause extensive abrasion on some of the equipment [15,71].
2.2.2. Breaking and Winnowing
Processing cocoa beans into semi-finished products, such as cocoa liquor, powder, and butter, requires the use of the essential part of the beans: the nibs (cotyledon). Thus, the nibs need to be separated from the shells. Breaking and winnowing entail applying pressure on the cocoa beans to loosen the shells (breaking) and subsequently separating the shells from the nibs so that only the nibs remain (winnowing). A thorough separation of the shells from the nibs is necessary to ensure a high-quality product devoid of a bitter and unpleasant taste with a gritty texture. Breaking and winnowing can be performed after roasting the beans (the traditional process) or before roasting. When the latter is performed, the nibs can easily be treated with an alkali solution (alkalization) [72].
2.2.3. Sterilization
Cocoa beans are exposed to microbial contamination during the primary processes of fermentation, drying, bagging, and transportation. Cocoa beans or nibs are thus subjected to saturated steam under pressure for a sufficiently long time to reduce the microbial load [15]. This process is referred to as sterilization and can be performed before or after roasting. In the latter situation, sterilization after roasting is used to ensure the destruction of heat-resistant bacteria and spores that might have survived the high temperatures of the roasting process [15,73]. During sterilization, an exposure time of seconds, rather than minutes, in the hot, low-pressure steam is sufficient to sterilize the bean [73]. This might be insufficient to cause any change in the flavanols and methylxanthine content. No work has reported changes in the flavanols and methylxanthine contents during sterilization.
2.2.4. Alkalization (Dutching)
Alkalization, also known as Dutching, is an optional step in the processing chain where cocoa nibs are treated with an alkali solution, such as potassium bicarbonate or sodium bicarbonate, to increase the pH to 7–8 with the purpose of modifying the color and taste and improve the dispersibility of the powder solids in water [15,20,45,54,74]. Alkalization has been found to influence the composition of the flavonoids and methylxanthines in cocoa products [45,75]. Alkalization has been found to result in the oxidation and polymerization of flavonoids, leading to a reduction in the astringency in alkalized cocoa nibs and powder [20,47]. High losses of polyphenols and changes in their composition have been reported in alkalized cocoa nibs and powder [20,42]. Work by Urbańska et al. [42] observed over a 60% loss of total polyphenols, and according to Giacometti et al. [47], the greatest losses were observed for epicatechin and catechin (up to ca. 98 and 80%, respectively) as well as for quercetin (ca. 80%). Earlier studies by Andres-Lacueva et al. [24] reported a reduction of 67% of epicatechin and 38% of catechin in cocoa powder after alkalization. Alkalization has also been found to decrease the amount of methylxanthine in the cocoa nib, thereby reducing the bitterness of alkalized cocoa powder [20,21,53]. The reduction in the methylxanthine content increases with an increasing degree of alkalization, and Li et al. [38] observed the greatest losses for theobromine (over 20%).
Alkalization can be performed in three main ways which include: nib alkalization, cake alkalization, and liquor alkalization [72,76]. Nib alkalization is the most common alkalization method, and it involves subjecting the cocoa nibs to an alkali treatment before the roasting and subsequent milling into liquor [72]. In cake alkalization, the cocoa nibs are first roasted and milled into liquor. The liquor is then pressed to extract the cocoa butter and the resultant cake is then kibbled (i.e., broken down into gravel-sized pieces) and subjected to an alkali treatment in a reaction vessel. Liquor alkalization involves subjecting the cocoa liquor to an alkali treatment to modify the color and flavor.
2.2.5. Cocoa Bean Roasting
Cocoa bean roasting involves subjecting the dried fermented cocoa beans to high temperatures, usually between 120 and 150 °C [47,77,78] for 15–45 min [79], and it is an important technological step that results in the production of flavor and aroma compounds, as well as the color changes that are desirable in cocoa products [6,42,77,80]. The time–temperature combination used in roasting depends on factors such as the type of cocoa (Criollo or Forastero), the intended final product, and the cocoa material (whole beans, nibs, or liquor) [69]. Several physiochemical changes occur during cocoa bean roasting due to the heat penetration into the beans. These changes include the loss of moisture from the beans (to ~2%), evaporation of volatile acids that contribute to acidity and bitterness, and loosening of the bean structure, which facilitates the removal of the shells and pressing of the butter during the liquor processing [15,42]. The heat also serves as a sterilization treatment which results in a further reduction in the number of microorganisms present in the beans [15,80].
Roasting significantly affects both the amount and composition of the polyphenols in cocoa beans [42,49,81]. It reduces the amounts of the total polyphenols, (−)-epicatechin, and proanthocyanidin in cocoa beans [50,51]. Żyżelewicz et al. [49], however, found increased catechin content during 20 min of roasting at 135 and 150 °C, which was attributed to the epimerization of the flavan-3-ol monomers and proanthocyanidins. Polyphenols are known to be thermolabile molecular compounds, and their content decreases with high temperatures and a prolonged roasting time. Ioannone et al. [82] believed that a low temperature and short roasting duration are better to preserve the polyphenolic content, with a concomitant improvement in the antioxidant activity of the roasted beans [51]. Temperatures below 140 °C have thus been recommended by Urbańska et al. [42] to preserve the polyphenolic content. Roasting has also been found to reduce the alkaloid content in cocoa beans. Aprotosoaie et al. [48] explained that during roasting, theobromine and caffeine could bond with diketopiperazines, resulting in a lower concentration of free alkaloids.
Cocoa beans can be roasted in three different ways, namely whole beans, nib, or liquor/mass roasting [15]. Cocoa beans are usually roasted using the whole-beans roasting method to produce the cocoa mass/liquor. This facilitates the removal of the shells during the subsequent breaking and winnowing stages. Nib roasting involves the removal of the shells before subjecting the nibs to roasting. Removing the shells before roasting enables the nibs to be treated with an alkali solution to change the color of the final cocoa powder or be treated with water or a sugar solution for flavor enhancement [15,20]. Liquor/mass roasting involves removing the shells and grounding them into liquor. The liquor/mass is then roasted, and the roasting can be performed using relatively inexpensive and simple equipment employing a scraped surface heat exchanger.
2.2.6. Cocoa Nib Grinding and Liquor Processing
Cocoa nibs are milled into a low-viscous mass referred to as cocoa liquor or cocoa mass. The viscosity of the liquor produced is greatly influenced by the degree of roasting and the moisture content of the nib. Cocoa liquor consists of the cocoa solid particles suspended in the cocoa butter [83]. The nibs are ground in a two-stage process: coarse grinding and fine grinding. The coarse or pre-grinding involves transforming the nibs from a solid to a thick paste, and this can be carried out in hammer mills, disc mills, or pin mills. Fine grinding, on the other hand, is performed to transform the thick paste into a smooth low-viscous mass, and it determines the end fineness and quality of the cocoa liquor. It is carried out in bead or ball mills. In small-scale processing, a kitchen blender can be used for coarse grinding, while the melanger is used for fine grinding. The desired end fineness of cocoa liquor is reported to be 15–70 μm [84] or 99.5% of the particles should be ≤75 μm [85]. Grinding cocoa nibs into liquor involves the application of shear under a controlled temperature. Changes in the polyphenols and methylxanthine contents during grinding have not been reported. Future studies can focus on the effects of the coarse and fine grinding of cocoa nibs on the polyphenols and methylxanthine composition of the final liquor produced. Cocoa liquor can be used directly in the manufacture of chocolate or processed by pressing to obtain two main products—cocoa butter and cocoa pressed cake (with a fat content between 10 and 24%) [15]. The extracted cocoa butter is used in the manufacture of chocolate and pharmaceutical and cosmetic products, whereas the cocoa press cake is further broken down into kibbles and pulverized to form cocoa powder. The pulverized cocoa powder is sieved to obtain a powder with a standardized particle size which is then used as the main ingredient in cocoa drinks and/or chocolate-flavored beverages. Anoraga et al. [86] noted that the yield of cocoa liquor pressing was influenced by the temperature, cocoa beans moisture content, particle size, and pressing time. However, the extent to which the bioactive compounds in cocoa liquor are affected during pressing remains unclear as no study has reported on it yet.
2.3. Chocolate Production
Chocolates are semisolid suspensions of fine solid particles from sugar and cocoa (and milk solids, depending on the type) in a continuous fat phase [15]. The conventional or industrial chocolate manufacture generally consists of the mixing of ingredients (the cocoa liquor/masse, sugar, cocoa butter, and milk component, depending on the chocolate type), refining (to reduce the particle size of the mixed chocolate paste), conching of chocolate paste, tempering (to form the stable fat crystals in the cocoa butter), casting and molding, cooling and de-molding, and finally, wrapping and packaging. The quality characteristics, such as the melting and rheological behavior, flavor, and sensory perception of chocolates as well as the bioactive compounds compositions, are influenced largely by the ingredient composition and processing method used [87].
One of the chocolate processing steps which could have a probable influence on the composition of the bioactive compound is conching. Conching consists of the mixing, shearing, and aeration of the chocolate mass during heating at a certain temperature (usually > 40 °C) to produce liquid chocolate, where all the solid particles are coated with fat [48,88]. It is an important stage for improving the quality of the chocolate. The main purpose of the conching process is to thoroughly combine all the ingredients to obtain a homogeneous mass [89]. It is also performed to evaporate the residual volatile compounds (e.g., acetic acids) and moisture to improve the color, texture, and flow characteristics of the chocolate mass [90]. Different time/temperature combinations are used in the conching process, depending on the chocolate type. Konar et al. [91] and Owusu et al. [92] have noted that temperatures ranging from 70 to 90 °C can be used to conch dark chocolates, while other studies have reported conching temperatures between 50 and 60 °C for milk chocolates to avoid the formation of Maillard compounds [89,93,94].
Limited studies have been conducted on the influence of conching on the polyphenolic and methylxanthine content of chocolates and their antioxidant properties, with varying opinions. Barišić et al. [52] noted that volatile polyphenols are lost during the initial stage of conching due to evaporation, together with water and short-chain fatty acids. Earlier studies by Afoakwa et al. [56] established that the content of the volatile polyphenols is reduced by 80% in this process. Sulistyowati and Misnawi [55] also observed a significant decrease in the concentration of the polyphenol and antioxidant activity due to the conching temperature. However, other studies found no significant variations (3%) in the phenolic content and pattern, as well as the antioxidant activity during the conching, regardless of the time/temperature combination applied [58,59,60]. The same results were reported by Di Mattia et al. [57] for the total polyphenol content.
Tempering is one of the most critical processing steps for making quality chocolate. It involves cooling while stirring the chocolate mass derived from the conching (from 40–50 to 18–28 °C) to obtain a stable form of the crystalline fat (polymorphic form V) responsible for the good melting properties and the glossy surface of good-quality chocolate [42,95]. However, changes in the polyphenols and methylxanthine contents at this stage have not been reported. Studies are needed to understand the influence of tempering on the phenolic and methylxanthine composition of chocolates.
3. Mechanisms of Action of Cocoa Flavanols
3.1. Recent Advances in Cocoa Flavanols on Signaling Pathways
In the last two decades, an emerging topic has been the study of the effect of flavonoids on MAPK and upstream/downstream-related proteins, receptors, or enzymes that control the cellular processes, such as proliferation, differentiation, apoptosis, and stress responses, under both normal and pathological conditions. MAPK signaling is activated in response to intra- and extracellular signals that activate the transmembrane glycoproteins of the tyrosine kinase receptor type, leading to the regulation of target genes. Three MAPK families have been reported in mammalian cells: extracellular signal-regulated kinase (ERK), c-Jun N terminal kinase/stress-activated protein kinase (JNK/SAPK), and p38 kinase [6,7,8]. The cyclic AMP-responsive element-binding protein 1 (CREB), a ubiquitous transcription factor, is activated by phosphorylation and is involved in the regulation of many cell processes [12,13,14]. The family of PI3Ks are enzymes involved in cell growth, proliferation, differentiation, survival, and intracellular trafficking. The activation of PI3K generates phosphatidylinositol (3,4,5)-trisphosphate (PIP3) and phosphatidylinositol (3,4)-diphosphate. Upon activation by upstream effectors such as PIP3 or phosphoinositide-dependent kinase-1 (PDK1), Akt translocates to the cell membrane and induces an enhancement of the mammalian target of rapamycin (mTOR) and the stimulation of cellular activities. Another family of signaling proteins involved in metabolic regulation that are regulated by flavonoids is the sirtuins (SIRT), especially SIRT1, that function as histone deacetylases [12,13,14].
The specific effect of cocoa flavanols, catechins, and procyanidins on MAPKs and PI3K/Akt signaling pathways has been frequently reported in different cell types and tissues [96]. Pioneer studies showed that epicatechin plays a role in liver cell survival, partially mediated by the induction of the AKT/PI-3-kinase and ERK1/2 pathways at micromolar concentrations [97,98]. A similar result was observed when a phenolic extract of cocoa was tested; thus, cocoa flavanols upregulated the antioxidant enzyme activity via the ERK1/2 pathway to protect against oxidative stress-induced apoptosis in hepatoma HepG2 cells [99]. Simultaneously, the same authors demonstrated that epicatechin-induced NF-κB, activator protein-1 (AP-1), and nuclear transcription factor erythroid 2p45-related (Nrf2) via the PI3K/AKT and ERK signaling in the same cell line [100]. A year later, the same group showed that cocoa procyanidin B2 and a cocoa polyphenolic extract inhibited the acrylamide-induced apoptosis in human colonic Caco-2 cells by the activation of the JNK pathway [101]. In the same human colonic cells, procyanidin B2 induced the Nrf2 translocation and glutathione-S-transferase P1 expression via ERKs and p38-MAPK pathways [102]. A year later, it was reported in cultured liver hepatoma cells that cocoa flavonoids improved the insulin signaling and repressed the glucose production [103] and protected those cells against high glucose-induced oxidative stress [104] via AKT and AMPK.
Because pure phenolic compounds are unlikely to avoid metabolism before reaching the cells, the study of cocoa flavanols metabolites has been an emerging topic during the last decade, in particular colonic microbiota metabolites from catechin and epicatechin. Thus, it has been later reported that microbial phenolic metabolites from cocoa flavanols improved the glucose-stimulated insulin secretion via ERKs and PKC pathways [105]. More recently, it has been reported that epicatechin and its colonic metabolite 3,4-dihydroxyphenylacetic acid protects the renal proximal tubular cell against high glucose-induced oxidative stress by modulating the NADPH oxidase (NOX)-4/SIRT1 signaling [106]. Most of that information regarding the ERK 1/2/CREB/TrkB-PI3K/Akt/mTOR signaling on all polyphenols, including cocoa flavanols, has been recently updated in comprehensive reviews [13,14,107]. New data have since been added to this particular topic that seem to mainly support previous evidence on the unambiguous effect of flavanols on these crucial signaling pathways. Thus, Camellia fascicularis extract, one of whose major components is epicatechin, could markedly inhibit the phosphorylation of p65, ERK, and JNK, thereby suppressing the activation of the NF-κB and MAPK signaling pathways, which may induce the secretion of pro-inflammatory cytokines [108].
Recent data also claim flavanols, through MAPK and related pathways, are potent inhibitors of neuroinflammation [109]. A recent study has reported that anti-inflammation properties of epicatechin on cultured macrophages were achieved, at least partly, by inhibiting the phosphorylation of the signal proteins (p65 and p38) involved in the MAPKs/NF-κB pathways [110]. In another recent study of cocoa flavanols, epicatechin and catechin have shown a significant docking capacity to TLR-4, JNK, NF-kB, and AP-1 through the formation of multiple hydrophilic and hydrophobic interactions [111]. Finally, epicatechin plays a protective effect on cardiac fibrosis, preventing myofibroblasts transformation, a process that involves the activation of the sumoylation of SIRT1 through SP1. Furthermore, SIRT1 inhibited the Ang II-induced fibrogenic effect via the AKT/glycogen synthase kinase (GSK3b) pathway [112]. Despite this plethora of data in support of the specific effect of flavanols on major signaling pathways and its potential preventive/therapeutic benefit in life-threatening diseases such as cancer or diabetes, the reported effect of cocoa flavanols has been inconceivably obviated in recent reviews on health effects of diet polyphenols [113,114,115,116,117,118].
3.2. Recent Advances in Cocoa Flavanols on Nrf2 Pathway/Antioxidant Defenses and Inflammatory Process
Although polyphenols exert their antioxidant capacity mainly through the direct neutralization of free radicals and chelating metals, such as Fe2+ and Cu+ [119,120], there are other mechanisms that have been reported, such as the stimulation of mitochondrial biogenesis through the activation of the SIRT1 and Nrf2 signaling pathways [120,121]. Nrf2, a transcription factor that regulates antioxidant responses, remains in the cytoplasm bound to the Kelch-like ECH-associated protein 1 (Keap1). Oxidative stress and some bioactive products disrupt the binding to Keap1 and release Nrf2 to translocate into the nucleus, where it binds to the antioxidant response element (ARE) in the promoter region of many antioxidant genes and initiates their transcription [121]. These antioxidant proteins include phase I antioxidant defense enzymes; phase II drug-metabolizing enzymes, such as glutathione-S-transferase (GST), NAD(P)H-quinone oxidoreductase-1 (NQO1), hemeoxygenase-1 (HO-1), and UDP-glucuronosyl transferase (UGT); or phase III transporters (multidrug resistance-associated proteins (MRPs) [121].
In particular, cocoa and its flavonoid compounds exert their protective effect against oxidative stress by targeting the transcription factor Nrf2 and Keap1, which participate in the regulation of the ARE. Thus, the regulation of Keap1 can lead to the nuclear accumulation of Nrf2 and the subsequent ARE activation [96]. The first reports on the role of cocoa flavanols on Nrf2 described that epicatechin increased the reduced glutathione (GSH) content, stimulated Nrf2 via the AKT/PKB in the astrocytes [122], and induced the Nrf2 translocation and phosphorylation in cultured hepatoma cells [100]. Similarly, procyanidin B2 evoked a dose-dependent increase in the glutathione peroxidase (GPx), glutathione reductase (GR), and GST, which improved the antioxidant response to an oxidative challenge in colonic Caco-2 cells [102]. In addition, procyanidin B2 induced the Nrf2 translocation and GST P1 expression to protect human colonic Caco-2 cells against oxidative stress [102]. Finally, catechin decreased the lipid peroxidation and reactive oxygen species (ROS) and increased the activity of GPx, the GR total sulfhydryl groups, and the expression of Nrf2 and heme oxygenase-1 in intestinal Int-407 cells [123].
Later, results on mouse cortical neuron cultures suggested that a combination of epicatechin and quercetin activated the Akt- and Ca(2+)-mediated signaling pathways that converge on nitric oxide synthase (NOS) and CREB; this effect results in synergistic improvements in the neuronal mitochondrial performance which confer the profound protection against ischemic injury [124]. Ramírez-Sánchez and colleagues [125] have demonstrated that epicatechin reverses the negative effects that high glucose or simulated type 2 diabetes has on NOS function in the diabetic heart. More recently, cocoa catechins were shown to improve the cellular redox state, resulting in the Nrf2 nuclear migration and upregulation of genes critical for mitochondrial respiration, glucose-stimulated insulin secretion, and ultimately improved the β-cell function [126]. Most of these previous results have been recently reviewed [127,128], although data concerning cocoa flavanols have been ignored in other reviews [129]. A recent study has reported that epicatechin reduced the cardiac fibrosis in an aged female rat model of pre-heart failure, which correlates with significant reductions in oxidative stress and cytokine levels in the absence of changes in contractile function [130]. Another study by Daussin et al. [131] also showed that the administration of cocoa flavanols to mice for 15 days stimulated the NAD metabolism which enhanced the SIRT metabolism and improved the mitochondrial function [131]. In addition, supplementation with a cocoa–carob blend diet rich in flavanols to Zucker diabetic fatty rats counteracted the oxidative stress in diabetic hearts by downregulating the NADPH oxidases, reducing the ROS generation, and modulating the SIRT1/Nrf2 signaling pathway, overall improving the antioxidant defense. Moreover, the supplemented diet suppressed the inflammatory and fibrotic reactions by inhibiting the NF-kB and pro-inflammatory and pro-fibrotic cytokines [132]. While testing a flavanol-rich cocoa supplementation during training on exercise performance, the results showed that the oxidative stress was lower in the cocoa-treated group than in the control group, together with lower interleukin-6 levels, an effect that might be mediated by the decrease in the expression of nuclear factor Nrf2 [133]. Finally, the administration of a 10% cocoa-enriched diet for 25 days to rats was able to prevent the excessive oxidative stress induced by intensive exercise, although it was not enough to avoid the immune function impairment due to exercise [120].
3.3. Recent Advances in Cocoa Flavanols on Cognitive Function
Cognitive function is defined as the mental performance that enables information processing, applying knowledge, and changing preferences [134]. Cognitive capacities, especially memory, attention, execution, and processing speed, gradually deteriorate throughout the adult lifespan, and lifestyle tactics such as diet may represent a favorable opportunity to delay or prevent the progressive cognitive decline [135,136]. In this context, numerous pieces of evidence from human clinical studies have strongly suggested that cocoa and cocoa-derived product consumption can be an effective, safe, and attractive approach to improving general cognition and working memory, especially among older people at risk or with cognitive decline [137,138].
Perhaps the first approach in the field was the finding of the uptake and metabolism of epicatechin and its access to the brain after oral ingestion [139]; then, the effect of flavanol-rich cocoa on the functional magnetic resonance imaging (fMRI) response to a cognitive task in healthy young people was reported [140]. Upon the discovery of the translational control by MAPK signaling in long-term synaptic plasticity and memory [141], Schroeter and colleagues [142] observed that epicatechin stimulated an ERK-dependent cyclic AMP-response element activity in the cortical neurons; this positive effect of epicatechin on MAPK was later confirmed in other tissues [104]. Perhaps due to this molecular mechanism, intervention assays in humans, such as the CoCoa Study, showed significant benefits in the cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment [143]. A year later, in a randomized controlled trial (RCT), a very low dose of cocoa polyphenols enhanced positive mood states but not cognitive performance [144], and a positive effect of flavanol-rich cocoa on cognitive capacity was further observed on cerebral perfusion in healthy older adults during the conscious resting state [145]. Considering all previous data, it is now widely assumed that cocoa monomeric flavanols, catechin, epicatechin, and their microbial metabolites, cross the blood–brain barrier and localize in the brain areas connected to learning and memory, such as the hippocampus, cerebral cortex, cerebellum, and striatum, which could potentially lead to cognitive enhancement. All these reports were exhaustively reviewed in 2020 [146,147,148,149,150,151] and more recently last year, with a focus on the molecular mechanisms and cognitive endpoints [13].
Ever since these comprehensive reviews, some new studies have mostly confirmed or substantiated the beneficial effect of cocoa flavanols on cognitive function. Epicatechin supplementation prevented short-term recognition memory impairment in high-fat diet-induced obese mice [152]. In a human study with healthy male volunteers, acute flavanol intake improved the efficiency of blood oxygenation (amplitude and speed) during hypercapnia in the frontal cortical areas of young healthy subjects; this is likely to contribute to improvements in cognitive function, but only when cognitive demands are high [153]. Additionally, in a crossover RCT, where 30 healthy men ingested a cocoa flavanol beverage (high-flavanol 150 mg vs. low-flavanol < 4 mg epicatechin) 1.5 h before an 8 min mental stress task, the flavanols were effective at counteracting the mental stress-induced endothelial dysfunction and improving the peripheral blood flow during stress [154]. In a recent comparative study between (−) and (+) epicatechin, on the mouse frontal cortex, both enantiomers, but more effectively (+)-epicatechin, upregulated the neurogenesis markers, likely through the stimulation of the capillary formation and NO triggering, resulting in improvements in memory [155]. Earlier this year, a randomized, double-blind, parallel-group study was performed on 60 healthy volunteers between 50 and 75 years old who consumed cocoa powder, and the results showed an improvement in executive function, without any change in neurotrophin levels [156]. In a recent review on cocoa flavanols and the aging brain, the authors concluded that neuropsychological ameliorations after cocoa intake were preceded by increases in the cerebral blood flow [157]. These results are in line with those of animal experimentation because improvements have been found in the motor and spatial performances of young and aging mice or rats as well as animal models of Alzheimer’s disease and Parkinson’s disease [158]. Considering that normal age-related memory decline is now considered an impending cognitive epidemic, dietary cocoa flavanols may offer meaningful benefits to cognitive health.
Nonetheless, cocoa/flavanol/chocolate treatment has also proved ineffective for cognitive function in some recent studies. In an eight-week RCT (FlaSeCo study), the short-term use of dark chocolate naturally high in flavanols showed no benefit in the studied cognitive parameters in cognitively healthy older adults [159]. In addition, the most recent clinical assay, a COSMOS-mind study with over 5000 participants, has concluded that a daily intake of cocoa extract for 3 years had no effect on cognition [160]. Moreover, over a median follow-up of 11.8 years, the results of the Framingham offspring cohort failed to declare a clear association between flavanol intake and a slower decline in cognitive function [161]. Despite these disappointments, most recent reviews have strongly claimed an overall enhancement of cognitive function by cocoa flavanols. In a systematic review/meta-analysis published in 2021, Gardener and colleagues [162] reported that of the 15 studies reviewed, 11 (2 observational studies, 6 chronic, and 3 acute intervention studies) found improvements in at least one cognitive domain following flavanol consumption. Of these 11 studies, flavanol intake was associated with improvements in global cognition as well as the cognitive domains of visual-spatial memory and organization, working memory, abstract reasoning, accuracy, reaction time, executive function, episodic memory, verbal fluency, and recognition memory [163]. A comparable conclusion is reached in recent systematic reviews and meta-analyses on polyphenols and cognition in humans [163]. It seems that cocoa’s long-term cognitive protection could particularly affect populations at risk or with early cognitive decline compared to old people who are cognitively intact [164,165]. However, we should bear in mind the fact that sugar content in chocolate and cocoa products is in general not declared and could in fact be a confounding factor in neurocognition studies. Sugar could affect cognition function and thus the inclusion of sugar-controlled studies would be desirable.
3.4. Recent Advances in Cocoa Flavanols in Cardiovascular Function
Perhaps the best established benefit of cocoa flavanols on health is their positive effect on cardiovascular function; in fact, the European Food Safety Authority (EFSA) has published two claims in support of the bioactivity of cocoa flavanols: cocoa flavanols help maintain normal blood pressure [166] and endothelium-dependent vasodilation [167], which contribute to normal blood flow. Moreover, to obtain the beneficial effect, a daily intake of 200 mg of cocoa flavanols is recommended, a quantity provided by 2.5 g of high-flavanol cocoa powder or 10 g of high-flavanol dark chocolate, both of which can be consumed in the context of a balanced diet. A recent meta-analysis provides evidence that cocoa flavanols could significantly improve endothelial function, with an optimal effect observed with 710 mg total flavanols, 95 mg (−)-epicatechin, or 25 mg (+)-catechin [168].
An overall agreement on the beneficial effects of cocoa flavanols on cardiovascular function has been proclaimed since the pioneer studies from two decades ago [169,170,171,172,173,174,175], through the first human trials [176,177,178] and first meta-analysis of controlled trials [179,180,181], up to the recent reviews [182,183,184,185,186,187,188]. Actually, a recent systematic review, meta-analysis, and dose–response analysis of RCTs have shown that the chronic consumption of dark chocolate and flavanols increased the flow-mediated dilatation (FMD); also, the acute consumption of dark chocolate and both dark chocolate and flavanols had beneficial effects on the FMD. The consumption of more than 40 g/day of dark chocolate increases the FMD with the highest mean of FMD in doses around 40–60 g/day [176]. In addition, a more recent review and dose–response meta-analysis of RCTs indicated the beneficial effect of the acute and chronic consumption of cocoa-based products ingestion on platelet function and arterial stiffness in healthy adults, regardless of age and the pattern of consumption (4 weeks) in the chronic intake (4 weeks) and in the acute intake (120 min) [189]. However, not all data have been so positive. Last year, a meta-analysis restricted to diabetic patients suggested that there is weak evidence for a reduction in diastolic but not systolic blood pressure after mid/long-term cocoa flavanol administration. These effects seem stronger in females, younger, and hypertensive when cocoa flavanols are ingested in one daily intake and when the epicatechin content is high enough [190].
Within the very last years, some new data have been added to the general knowledge; thus, an acute crossover RCT was conducted in 20 healthy males, adding further evidence that epicatechin is a causal vasoactive molecule within flavanol-containing foods/beverages. Interestingly, the data revealed that intake levels as low as 0.5 mg/kg body weight are capable of inducing acute improvements in vascular function, evaluated as the FMD, in healthy volunteers [191]. In the same line, in a cohort of healthy young and elderly subjects, the twice-daily consumption of 450 mg of cocoa flavanols during a two week period decreased the endothelial compared to the cocoa flavanol-free control. This decrease is inversely correlated with FMD improvements, which indicates that flavanol consumption can improve the endothelial functional integrity in healthy humans [192]. Moreover, data from 25,618 participants of the European Prospective Investigation into Cancer (EPIC) Norfolk cohort showed that hypertensive participants had a stronger inverse association between flavanol biomarkers and systolic blood pressure when compared to normotensive participants. Therefore, flavanol intake could have a role in the maintenance of cardiovascular health in the general population [193]. In a trial published early this year, 20 healthy middle-aged men consumed cocoa flavanols (twice daily, 450 mg) or control drinks for 1 month, and the results showed that flavanol consumption may mediate the vascular protective effects by modulating the gene expression and DNA methylation and by preserving the integrity of the immunological–endothelial barrier functions [194]. Moreover, in this current year, an RCT with cocoa extract supplementation (500 mg flavanols, of which included 80 mg epicatechin, daily) among 21,442 US adults reported that cocoa extract supplementation did not significantly reduce the total cardiovascular events among older adults but reduced the cardiovascular disease death by 27% [195].
However, not all the studies have shown beneficial effects; thus, a recent meta-analysis showed a significant inverse association between cocoa consumption and systolic/diastolic blood pressure, but the analysis could not conclude any beneficial effect of cocoa consumption on blood pressure in normotensive/elevated blood pressure subjects [196]. Moreover, 200 mg daily of monomeric and oligomeric flavanols for three months on top of habitual diet and usual care did not reduce the plasma markers of endothelial dysfunction compared to the placebo in patients with long-term type 2 diabetes [197]. Finally, early this year, a crossover trial with type 2 diabetic (T2DM) and non-diabetic subjects that received 790 mg of cocoa flavanols daily reported that no beneficial effects of cocoa flavanols were detected on the vascular reactivity parameters in T2DM and non-diabetic participants [190].
4. Mechanisms of Action of Cocoa Methylxanthines
4.1. Recent Advances in Mechanisms of Action of Cocoa Theobromine
Methylxanthines show important biological activities that have several benefits for human health, acting against respiratory and cardiovascular diseases, cancer, obesity and diabetes, human infertility, neurological, and neurodegenerative diseases [198,199]. Theobromine (3,7-dimethylxanthine) is the main alkaloid of cocoa and its biological effects have been usually considered secondary to those of the main cocoa polyphenols, flavanols. However, recent studies in rats fed with cocoa or only theobromine have reported that this methylxanthine is the main factor responsible for cocoa’s effects on body weight gain as well as on lipid and glucose metabolism [200]. This and other results have raised interest in the study of the cocoa compound and synthetic chemical derivatives, such as pentoxifylline, formed by introducing a hexanone group to the theobromine molecule. This derivative is a non-selective phosphodiesterase inhibitor that has shown promising results, and it is being widely used as a therapeutic agent [201]. Some of the advances in the knowledge of the biological effects and mechanisms of action of theobromine are discussed below.
4.2. Recent Advances in Theobromine on Signaling Pathways
Many reported effects of theobromine are related to its role as a phosphodiesterase inhibitor. In high concentrations, which cannot be reached by nutritional intake but through pharmaceutical administration, theobromine and other methylxanthines lead to the inhibition of cyclic nucleotide phosphodiesterases and high-affinity ATP-dependent cyclic nucleotide transporters. This inhibition results in an increase in the cellular cyclic AMP levels and mobilization of intracellular calcium and, as a consequence, a modulation of the gamma-aminobutyric acid (GABA) receptors [202]. Still, in the last century, a pioneer study reported that theobromine acted as a purinergic receptor antagonist on tumor-induced angiogenesis in BALB/c mice [203]. The results of this study suggested that theobromine reduces neovascularization, tumor progression, and metastasis via the adenosine A2a receptor inhibition in ovarian cancer cells. Some years later, but still within the last century, another study described the influence of theobromine on the angiogenic activity and proangiogenic cytokines production of human ovarian cancer cells [204]. A later study showed that theobromine prevented malignant glioblastoma proliferation by negatively regulating phosphodiesterase-4, extracellular signal-regulated kinases (ERK), Akt/mTOR kinase, and nuclear factor-κB (NF-κB) [205]. A year later, new data suggested that theobromine inhibited the adipocyte differentiation during the early stages of adipogenesis by regulating the expression of peroxisome proliferator-activated receptor (PPAR)γ and CCAAT/enhancer-binding proteins (C/EBPs)α through the AMPK and ERK/JNK signaling pathways in the 3T3-L1 preadipocytes [206]. Simultaneously, theobromine was observed to increase the NAD+/SIRT1 activity protecting the kidney under diabetic conditions [207].
Two years later, a study reported that theobromine presented anti-proliferative activity against colorectal cancer cells HT-29 by modulating the gene expression associated with cell growth pathways. In fact, the Bax/Bcl-2 ratio was strongly upregulated in cells exposed to theobromine, significantly decreasing the cell proliferation [208]. The following year, Yoneda and colleagues showed that theobromine upregulates the cerebral brain-derived neurotrophic factor (BDNF) and augmented the cAMP/CREB/BDNF pathways and motor learning in mice [209]. Still, in 2017, theobromine suppressed the adipocyte differentiation and induced the C/EBPβ degradation by increasing its post-translational modification by sumoylation. Furthermore, it has been shown that the inhibition of AR1 signaling is important for theobromine-induced C/EBPβ degradation [210]. In a recent study, theobromine inhibited dimethylhydrazine-induced colon cancer through the downregulation of the Akt/mTOR and JAK2/STAT3 pathways and an increment of the Smad2 tumor suppressor [211]. Additionally, theobromine supplementation upregulated multiple thermogenic adipocyte marker genes in subcutaneous adipose tissue. Furthermore, in mouse-derived primary adipocytes, theobromine upregulated the expression of the uncoupling protein (UCP)1 and mitochondrial mass in a peroxisome proliferator-activated receptor γ (PPARγ) ligand-dependent manner, and it also increased the phosphorylation levels of the PPAR γ coactivator 1 α. These results indicate that dietary supplementation with theobromine induces browning in subcutaneous white adipose tissue and suggests its potential to treat obesity [212].
Theobromine downregulated the sterol regulatory element-binding protein (SREBP)-1c, fatty acid synthase (FASN), and upregulated the PPARα and CPT1a mRNA and protein levels in cultured hepatocytes and improved non-alcoholic fatty liver disease by inhibiting lipogenesis and fatty acid uptake and promoting fatty acid oxidation in the liver, which might be associated with its suppression of the mTOR signaling pathway [213]. Recent research provided evidence that methylxanthines can induce changes in lipid profiles that might be beneficial with respect to neurodegenerative diseases, such as Alzheimer’s and other diseases affected by lipid alterations [214]. Similarly, theobromine, from the cocoa shell, enhanced the ERK1/2 phosphorylation and fibroblast growth factor (FGF)21 release via PPAR activation. The increase in the phosphorylation of the insulin receptor, AKT, AMPK, mTOR, and ERK1/2 conduced to the regulation of hepatic mitochondrial function and energy metabolism [215]. Regarding the redox status and inflammatory process, a motivating study from 2015 in mice fed a theobromine-rich diet for 20 days showed that the main methylxanthine of cocoa is able to stimulate the Nrf2 activation and, consequently, the expression of both superoxide dismutase (SOD)-1 and GPx by itself [216]. More recently, theobromine displayed a preventive effect against interleukin (IL)-1β-induced chondrocyte dysfunction through the reduction in the IL-1β-induced production of cellular ROS and inflammatory mediators, including ciclooxigenase 2 (COX-2), prostaglandin E2, and NF-KB [217].
4.3. Recent Advances in Cocoa Theobromine on Cardiovascular Function
One of the first reports showed that the acute consumption of high-flavanol/high-theobromine chocolate increased the plasma epicatechin and theobromine concentrations and decreased the arterial stiffness, with no effect on the endothelial function and a marginal increase in the diastolic blood pressure. However, the chronic intake increased the plasma theobromine, though it did not have positive impacts on the endothelial function, arterial stiffness, or blood pressure in pregnant women at risk of preeclampsia [8]. On the other hand, the results show that the regular consumption of a theobromine/flavanols-rich cocoa product may contribute to the changes in cholesterol (and indirectly the HDL cholesterol) observed after the regular intake in healthy and cardiovascular risk subjects. The data also suggest that theobromine and 7-methylxanthine (the main theobromine metabolite), together with its content in insoluble dietary fiber, may be responsible for the decrease in IL-1β and the hypoglycemic effects observed [218]. In a study from last year, theobromine protected against the glutamate toxicity-induced GABAergic decline in the brain of rats with transient global cerebral ischemia injury. These findings suggested that theobromine could alleviate the chances of stroke by preventing acute episodes of cerebral ischemia [9].
Other authors observed a reduction in the brain oxidative stress, inflammatory intermediaries (tumor necrosis factor α (TNF-α), interleukin-1β, and -6, NF-κB), markers of cell damage (lactate dehydrogenase and caspase-3), acetylcholinesterase activity, and improvement in γ-aminobutyric acid quantity in rats that were given theobromine for 14 days daily after cerebral hypoperfusion [10]. Similarly, theobromine, as the most abundant component of a cocoa shell extract, showed vasodilator properties associated with increased NO bioavailability, suggesting that such a cocoa by-product is a potential fool ingredient for diseases related to endothelial dysfunction [219]. However, not all studies have supported the beneficial effects of theobromine or its derivatives. Thus, trying to decipher whether the beneficial effects of cocoa were partly due to theobromine, Talbot and coworkers [220] designed an RCT with 30 overweight and 14 obese men and women that consumed daily 500 mg of theobromine or placebo for 4 weeks. The goal was to unravel whether the effects of theobromine are mediated through the effects on HDL-mediated cholesterol efflux, which may be affected by the microRNA (miRNA) levels in the HDL particles. The results showed that theobromine did not improve the fasting and postprandial ATP-binding cassette subfamily A Member 1-mediated cholesterol efflux capacity but decreased the fasting miR-92a levels. The authors concluded that 4 weeks of theobromine consumption did not change the HDL-mediated cholesterol efflux capacity at baseline and postprandially [220]. In another study, theobromine consumption did not improve the fasting and postprandial endothelial function but increased the postprandial peripheral arterial diameters and decreased the augmentation index. These findings do not support the contribution of theobromine alone to the proposed cardioprotective effects of cocoa [221].
4.4. Recent Advances in Cocoa Theobromine on Cognitive Function and Other Effects
Until recently, cocoa’s effects on cognitive function were plainly associated with the polyphenolic fraction, especially flavanols [13]. In fact, most of the previous studies dealing with the effect of theobromine on cognitive and other brain functions failed to show significant changes [222]. However, it has been known for some years that theobromine blocks adenosine receptors to reduce the endogenous inhibitory adenosine and evoke a stimulatory effect on the CNS; thus, increased dopaminergic activity in the brain is suggested to mediate the psychostimulant effect [202]. This significant effect raised interest in the specific effects of theobromine independently from those already reported for flavanols.
The first encouraging results were published still in the last century. Pioneer studies showed that theobromine ingestion caused an increase in the brain levels of A1-adenosine receptors [223]. No more positive results were reported until ten years later when the administration of 30 mg/kg of theobromine to mice increased the ambulatory activity [224]. Some years later, in an animal experiment, Fernández-Fernández and coworkers [216] observed an enhanced modulatory effect on both cholinergic and catecholaminergic transmissions on mice fed 20 days with a theobromine-rich diet. The enhancing effect of theobromine on the levels of acetylcholine-related enzymes, dopamine, and especially noradrenalin confirms its beneficial role on the “cognitive reserve” and, consequently, a possible reducing effect on the cognitive decline underlying aging and Alzheimer’s disease [216]. In a study with volunteers to unravel the differential contributions of theobromine and caffeine on mood, psychomotor performance, and blood pressure, the authors concluded that caffeine might have more CNS-mediated effects on alertness, while theobromine might be acting primarily via peripheral physiological changes [225]. Moreover, in 2015, in a study of patients with Alzheimer’s disease, theobromine was found to be associated with a favorable Aβ profile in the cerebrospinal fluid [226].
Somewhat later, a study using a fat-enriched diet to induce a long-term deterioration in cognitive and memory functions showed that theobromine, at realistic concentrations in drinking water, restored the A1 receptor levels and improved the cognitive functions and Aβ levels [227]. Still, in 2017, the results in experimental animals strongly suggested that in mice orally administered 0.05% theobromine for 30 days, a phosphodiesterase inhibitor effect in the brain was produced, and it augmented motor learning through the cAMP/CREB/BDNF pathways (see also the section on signaling pathways), which play a crucial role in memory and learning [209]. In a comprehensive review from 2019 exploring the potential of theobromine as a cognitive regulator, the authors concluded that animal and human studies suggested a potential neuroprotective action of the long-term consumption of theobromine through a reduction in the Aβ amyloid pathology, which is observed in Alzheimer’s disease patients’ brains [222]. Early this year, treatment with theobromine significantly attenuated the neurological deficits and improved the sensorimotor functions and memory in rats with cerebral hypoperfusion. The authors suggested that the findings in the parameters regarding the oxidative stress, inflammatory intermediaries, and histopathological analysis substantiated the attenuation of neurodegenerative changes by theobromine [10]. Moreover, in this current year, a cross-sectional study on a representative American population aged ≥60 years has shown that a daily intake of theobromine tended to be associated with a better cognitive performance [11].
Regarding other biological effects recently attributed to theobromine, it is worth mentioning that methylxanthine plays a relevant role in some effects related to cocoa intake, such as the lower proportion of IgA-coated bacteria. Moreover, theobromine modifies the gut microbiota, although other cocoa compounds could also act on intestinal bacteria, attenuating or enhancing the theobromine effects [228]. Finally, research from this current year supports the use of methylxanthines, in particular theobromine, as a SARS-CoV-2 inhibitor as compared to chloroquine [229].
5. Conclusions
Cocoa bean processing is important as it improves both the economic and technological values of the beans. The primary and secondary processing of cocoa develops the characteristic flavor and color desired in chocolate and other cocoa-related products. However, processing, including chocolate production, affects both the composition and quantities of the flavanols and methylxanthines, mainly the theobromine. Cocoa bean processing mainly results in the reduction in the flavanols and theobromine content of cocoa beans and cocoa-related products. This modifies the taste and flavor as well as the health-promoting properties of cocoa beans and chocolates. Most of the work conducted on the influence of processing on the flavanols and methylxanthine contents in cocoa have focused mainly on the primary processes, with few processing steps during the industrial processing (mainly roasting and alkalization). No studies have reported on the impact of cocoa nib grinding, liquor pressing, and chocolate tempering on the composition of the bioactive compounds. Limited studies have been reported on the influence of conching. Considering the increased consumer interest in cocoa and cocoa-related products due to the presence of bioactive compounds, and the effect processing might have on their bioactivity and mechanisms of action, a holistic study of the entire processing steps (from beans to chocolates and other related products) and its impact on the bioactive compounds would have significant technological and commercial implications. Again, most of the studies reviewed here deal with cocoa beans, cocoa shells, cocoa powder, or black high-cocoa chocolates in which mainly flavanols and theobromine coexist. In this sense, it is challenging, in most cases, to attribute the activity and the mechanisms of action to one fraction or the other. However, we can conclude that theobromine seems to be effective, mainly through the regulation of the fatty acid metabolism, mitochondrial function, and energy metabolism pathways. Flavanols, however, seem to work through the protein kinases MAPKs and PI3K/Akt signaling pathways but also by regulating oxidative stress via targeting the transcription factor Nrf2 and Keap1, which participate in the activation of the antioxidant response element. Both flavanols and theobromine might be responsible for the nitric oxide regulatory effect of cocoa and dark chocolate for the neurological activity through this pathway and the regulation of neurotrophic factors and neuroplasticity pathways.
Taking all of the above into account, it would be interesting to investigate the effects that the transformation of cocoa into chocolate produces on the original cocoa bioactive compounds and on the molecular mechanisms associated with their intake. On the other hand, there is a clear need to search for a final product that eliminates, if not completely, most of the sugar content that chocolates currently have on the market. This would allow for enhancing the health benefits associated with the consumption of chocolate by eliminating the risk associated with the consumption of added sugars and by selectively increasing the bioactive compounds of interest by modifying the technological food processes.
Author Contributions
Conceptualization, research, writing—review, and editing, J.E.K., L.G. and S.d.P.-T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Spanish MCIN through the Agencia Estatal de Investigación (AEI), grant number PID2019-107009RB-100/AEI/10.13039/501100011033, and the CSIC i-COOP program 2021, grant number COOPB20586.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Perez M., Lopez-Yerena A., Vallverdú-Queralt A. Traceability, authenticity and sustainability of cocoa and chocolate products: A challenge for the chocolate industry. Crit. Rev. Food Sci. Nutr. 2022;62:475–489. doi: 10.1080/10408398.2020.1819769. [DOI] [PubMed] [Google Scholar]
- 2.Ramos-Escudero F., Casimiro-Gonzales S., Fernández-Prior Á., Cancino Chávez K., Gómez-Mendoza J., de la Fuente-Carmelino L., Muñoz A.M. Colour, fatty acids, bioactive compounds, and total antioxidant capacity in commercial cocoa beans (Theobroma cacao L.) LWT Food Sci. Technol. 2021;147:111629. doi: 10.1016/j.lwt.2021.111629. [DOI] [Google Scholar]
- 3.Melo T.S., Pires T.C., Engelmann J.V.P., Monteiro A.L.O., Maciel L.F., Bispo E.D.S. Evaluation of the content of bioactive compounds in cocoa beans during the fermentation process. J. Food Sci. Technol. 2021;58:1947–1957. doi: 10.1007/s13197-020-04706-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samanta S., Sarkar T., Chakraborty R., Rebezov M., Shariati M.A., Thiruvengadam M., Rengasamy K.R.R. Dark chocolate: An overview of its biological activity, processing, and fortification approaches. Curr. Res. Food Sci. 2022;5:1916–1943. doi: 10.1016/j.crfs.2022.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rimbach G., Egert S., de Pascual-Teresa S. Chocolate: (un)Healthy source of polyphenols? Genes Nutr. 2011;6:1–3. doi: 10.1007/s12263-010-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kongor J.E., Hinneh M., Van de Walle D., Afoakwa E.O., Boeckx P., Dewettinck K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—A review. Food Res. Int. 2016;82:44–52. doi: 10.1016/j.foodres.2016.01.012. [DOI] [Google Scholar]
- 7.Quarmine W., Haagsma R., Sakyi-Dawson O., Asante F., van Huis A., Obeng-Ofori D. Incentives for cocoa bean production in Ghana: Does quality matter? NJAS Wagening J. Life Sci. 2012;60–63:7–14. doi: 10.1016/j.njas.2012.06.009. [DOI] [Google Scholar]
- 8.Babar A., Bujold E., Leblanc V., Lavoie-Lebel E., Paquette J., Bazinet L. Changes in endothelial function, arterial stiffness and blood pressure in pregnant women after consumption of high-flavanol and high-theobromine chocolate: A double blind randomized clinical trial. Hypertens. Pregnancy. 2018;37:68–80. doi: 10.1080/10641955.2018.1446977. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad Bhat J., Gupta S., Kumar M. Neuroprotective effects of theobromine in transient global cerebral ischemia-reperfusion rat model. Biochem. Biophys. Res. Commun. 2021;571:74–80. doi: 10.1016/j.bbrc.2021.07.051. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad Bhat J., Kumar M. Neuroprotective effects of theobromine in permanent bilateral common carotid artery occlusion rat model of cerebral hypoperfusion. Metab. Brain Dis. 2022;37:1787–1801. doi: 10.1007/s11011-022-00995-6. [DOI] [PubMed] [Google Scholar]
- 11.Gao L., Ge W., Peng C., Guo J., Chen N., He L. Association between dietary theobromine and cognitive function in a representative American population: A cross-sectional study. J. Prev. Alzheimer Dis. 2022;3:449–457. doi: 10.14283/jpad.2022.39. [DOI] [PubMed] [Google Scholar]
- 12.Cichon N., Saluk-Bijak J., Gorniak L., Przyslo L., Bijak M. Flavonoids as a natural enhancer of neuroplasticity. An overview of the mechanism of neurorestorative action. Antioxidants. 2020;9:1035. doi: 10.3390/antiox9111035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goya L., San Román R., de Pascual-Teresa S. Polyphenols’ effect on cerebrovascular health. Curr. Med. Chem. 2021;29:1029–1044. doi: 10.2174/0929867328666211129123459. [DOI] [PubMed] [Google Scholar]
- 14.Gasmi A., Mujawdiya P.K., Noor S., Lysiuk R., Darmohray R., Piscopo S., Lenchyk L., Antonyak H., Dehtiarova K., Shanaida M., et al. Polyphenols in Metabolic Diseases. Molecules. 2022;27:6280. doi: 10.3390/molecules27196280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afoakwa E.O. Chocolate Science and Technology. 2nd ed. Wiley-Blackwell; Oxford, UK: 2016. pp. 21–550. [Google Scholar]
- 16.Di Mattia C.D., Sacchetti G., Mastrocola D., Serafini M. From cocoa to chocolate: The impact of processing on in vitro antioxidant activity and the effects of chocolate on antioxidant markers in vivo. Front. Immunol. 2017;8:1207. doi: 10.3389/fimmu.2017.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Żyżelewicz D., Budryn G., Oracz J., Antolak H., Kręgiel D., Kaczmarska M. The effect on bioactive components and characteristics of chocolate by functionalization with raw cocoa beans. Food Res. Int. 2018;113:234–244. doi: 10.1016/j.foodres.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Muñoz M.S., Cortina J.R., Vaillant F.E., Parra S.E. An overview of the physical and biochemical transformation of cocoa seeds to beans and to chocolate: Flavor formation. Crit. Rev. Food Sci. Nutr. 2020;60:1593–1613. doi: 10.1080/10408398.2019.1581726. [DOI] [PubMed] [Google Scholar]
- 19.Hinneh M., Van de Walle D., Tzompa-Sosa D.A., De Winne A., Termote S., Messens K., Van Durme J., Afoakwa E.O., De Cooman L., Dewettinck K. Tuning the aroma profiles of FORASTERO cocoa liquors by varying pod storage and bean roasting temperature. Food Res. Int. 2019;125:108550. doi: 10.1016/j.foodres.2019.108550. [DOI] [PubMed] [Google Scholar]
- 20.Sharif S. Flavor development during cocoa roasting. In: Hii C.L., Borém F.M., editors. Drying and Roasting of Cocoa and Coffee. 1st ed. CRC Press—Taylor & Francis Group; Boca Raton, FL, USA: 2020. pp. 63–87. [Google Scholar]
- 21.Febrianto N.A., Wang S., Zhu F. Chemical and biological properties of cocoa beans affected by processing: A review. Crit. Rev. Food Sci. Nutr. 2021;62:1–32. doi: 10.1080/10408398.2021.1928597. [DOI] [PubMed] [Google Scholar]
- 22.Febrianto N.A., Zhu F. Changes in the composition of methylxanthines, polyphenols, and volatiles and sensory profiles of cocoa beans from the Sul 1 genotype affected by fermentation. J. Agric. Food Chem. 2020;68:8658–8675. doi: 10.1021/acs.jafc.0c02909. [DOI] [PubMed] [Google Scholar]
- 23.Júnior P.C.G., dos Santos V.B., Lopes A.S., de Souza J.P.I., Pina J.R.S., Júnior G.C.A.C., Marinho P.S.B. Determination of theobromine and caffeine in fermented and unfermented Amazonian cocoa (Theobroma cacao L.) beans using square wave voltammetry after chromatographic separation. Food Control. 2020;108:106887. doi: 10.1016/j.foodcont.2019.106887. [DOI] [Google Scholar]
- 24.Andres-Lacueva C., Monagas M., Khan N., Izquierdo-Pulido M., Urpi-Sarda M., Permanyer J., Lamuela-Raventós R.M. Flavanol and flavonol contents of cocoa powder products: Influence of the manufacturing process. J. Agric. Food Chem. 2008;56:3111–3117. doi: 10.1021/jf0728754. [DOI] [PubMed] [Google Scholar]
- 25.Eker M.E., Aaby K., Budic-Leto I., Rimac Brnčić S., El S.N., Karakaya S., Simsek S., Manach C., Wiczkowski W., de Pascual-Teresa S. A review of factors affecting anthocyanin bioavailability: Possible implications for the inter-individual variability. Foods. 2020;9:2. doi: 10.3390/foods9010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afoakwa E. Cocoa Production and Processing Technology. 1st ed. CRC Press; Boca Raton, FL, USA: 2014. pp. 1–374. [Google Scholar]
- 27.Krähmer A., Engel A., Kadow D., Ali N., Umaharan P., Kroh L.W., Schulz H. Fast and neat—Determination of biochemical quality parameters in cocoa using near-infrared spectroscopy. Food Chem. 2015;181:152–159. doi: 10.1016/j.foodchem.2015.02.084. [DOI] [PubMed] [Google Scholar]
- 28.Dand R. The International Cocoa Trade. 3rd ed. Woodhead Publishing; Sawston, UK: 2010. pp. 55–59. [Google Scholar]
- 29.Amoa-Awua W.K. Methods of cocoa fermentation and drying. In: Schwan R.F., Fleet G.H., editors. Cocoa and Coffee Fermentations. 1st ed. CRC Press—Taylor & Francis Group; New York, NY, USA: 2015. pp. 71–116. [Google Scholar]
- 30.Pereira-Caro G., Borges G., Nagai C., Jackson M.C., Yokota T., Crozier A., Ashihara H. Profiles of phenolic compounds and purine alkaloids during the development of seeds of Theobroma cacao cv. Trinitario. J. Agric. Food Chem. 2012;61:427–434. doi: 10.1021/jf304397m. [DOI] [PubMed] [Google Scholar]
- 31.Febrianto N.A., Zhu F. Diversity in composition of bioactive compounds among 26 cocoa genotypes. J. Agric. Food Chem. 2019;67:9501–9509. doi: 10.1021/acs.jafc.9b03448. [DOI] [PubMed] [Google Scholar]
- 32.Dang Y.K.T., Nguyen H.V.H. Effects of maturity at harvest and fermentation conditions on bioactive compounds of cocoa beans. Plant Foods Hum. Nutr. 2019;74:54–60. doi: 10.1007/s11130-018-0700-3. [DOI] [PubMed] [Google Scholar]
- 33.Zheng X.Q., Koyama Y., Nagai C., Ashihara H. Biosynthesis, accumulation and degradation of theobromine in developing Theobroma cacao fruits. J. Plant Physiol. 2004;161:363–369. doi: 10.1078/0176-1617-01253. [DOI] [PubMed] [Google Scholar]
- 34.Nazaruddin R., Seng L., Hassan O., Said M. Effect of pulp pre-conditioning on the content of polyphenols in cocoa beans (Theobroma cacao) during fermentation. Ind. Crops Prod. 2006;24:87–94. doi: 10.1016/j.indcrop.2006.03.013. [DOI] [Google Scholar]
- 35.Afoakwa E.O., Kongor J.E., Takrama J., Budu A. Changes in nib acidification and biochemical composition during fermentation of pulp pre-conditioned cocoa (Theobroma cacao) beans. Int. Food Res. J. 2013;20:1843–1853. [Google Scholar]
- 36.Sulaiman K.B., Yang T.A., Ariffin F. Colour and antioxidant properties of cocoa beans from pods storage and fermentation using shallow box. Int. J. Sci. Technol. 2017;3:455–465. doi: 10.20319/mijst.2017.32.455465. [DOI] [Google Scholar]
- 37.Aikpokpodion P.E., Dongo L.N. Effects of fermentation intensity on polyphenols and antioxidant capacity of cocoa beans. Int. J. Sustain. Crop Prod. 2010;5:66–70. [Google Scholar]
- 38.Suazo Y., Davidov-Pardo G., Arozarena I. Effect of fermentation and roasting on the phenolic concentration and antioxidant activity of cocoa from Nicaragua. J. Food Qual. 2014;37:50–56. doi: 10.1111/jfq.12070. [DOI] [Google Scholar]
- 39.Albertini B., Schoubben A., Guarnaccia D., Pinelli F., Della Vecchia M., Ricci M., Di Renzo G.C., Blasi P. Effect of fermentation and drying on cocoa polyphenols. J. Agric. Food Chem. 2015;63:9948–9953. doi: 10.1021/acs.jafc.5b01062. [DOI] [PubMed] [Google Scholar]
- 40.Oracz J., Żyżelewicz D., Nebesny E. The content of polyphenolic compounds in cocoa beans (Theobroma cacao L.), depending on variety, growing region, and processing operations: A review. Crit. Rev. Food Sci. Nutr. 2015;55:1176–1192. doi: 10.1080/10408398.2012.686934. [DOI] [PubMed] [Google Scholar]
- 41.Hernández-Hernández C., Viera-Alcaide I., Morales-Sillero A.M., Fernández-Bolaños J., Rodríguez-Gutiérrez G. Bioactive compounds in Mexican genotypes of cocoa cotyledon and husk. Food Chem. 2018;240:831–839. doi: 10.1016/j.foodchem.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 42.Urbańska B., Derewiaka D., Lenart A., Kowalska J. Changes in the composition and content of polyphenols in chocolate resulting from pre-treatment method of cocoa beans and technological process. Eur. Food Res. Technol. 2019;245:2101–2112. doi: 10.1007/s00217-019-03333-w. [DOI] [Google Scholar]
- 43.Camu N., De Winter T., Addo K.S., Takrama J.S., Bernaert H., De Vuyst L. Fermentation of cocoa beans: Influence of microbial activities and polyphenol concentrations on the flavour of chocolate. J. Sci. Food Agric. 2008;88:2288–2297. doi: 10.1002/jsfa.3349. [DOI] [Google Scholar]
- 44.Peláez P., Bardón I., Camasca P. Methylxanthine and catechin content of fresh and fermented cocoa beans, dried cocoa beans, and cocoa liquor. Sci. Agropecu. 2016;7:355–365. doi: 10.17268/sci.agropecu.2016.04.01. [DOI] [Google Scholar]
- 45.Gil M., Uribe D., Gallego V., Bedoya C., Arango-Varela S. Traceability of polyphenols in cocoa during the postharvest and industrialization processes and their biological antioxidant potential. Heliyon. 2021;7:e07738. doi: 10.1016/j.heliyon.2021.e07738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurst W.J., Krake S.H., Bergmeier S.C., Payne M.J., Miller K.B., Stuart D.A. Impact of fermentation, drying, roasting and Dutch processing on flavan-3-ol stereochemistry in cacao beans and cocoa ingredients. Chem. Cent. J. 2011;5:53. doi: 10.1186/1752-153X-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giacometti J., Jolić S.M., Josić D. Cocoa processing and impact on composition. In: Preedy V., editor. Processing and Impact on Active Components in Food. Academic Press; Cambridge, MA, USA: 2015. pp. 605–612. [DOI] [Google Scholar]
- 48.Aprotosoaie A.C., Luca S.V., Miron A. Flavor chemistry of cocoa and cocoa products—An overview. Compr. Rev. Food Sci. Food Saf. 2016;15:73–91. doi: 10.1111/1541-4337.12180. [DOI] [PubMed] [Google Scholar]
- 49.Żyżelewicz D., Krysiak W., Oracz J., Sosnowska D., Budryn G., Nebesny E. The influence of the roasting process conditions on the polyphenol content in cocoa beans, nibs and chocolates. Food Res. Int. 2016;89:918–929. doi: 10.1016/j.foodres.2016.03.026. [DOI] [Google Scholar]
- 50.Stanley T.H., Van Buiten C.B., Baker S.A., Elias R.J., Anantheswaran R.C., Lambert J.D. Impact of roasting on the flavan-3-ol composition, sensory-related chemistry, and in vitro pancreatic lipase inhibitory activity of cocoa beans. Food Chem. 2018;255:414–420. doi: 10.1016/j.foodchem.2018.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oracz J., Nebesny E. Effect of roasting parameters on the physicochemical characteristics of high-molecular-weight Maillard reaction products isolated from cocoa beans of different Theobroma cacao L. groups. Eur. Food Res. Technol. 2019;245:111–128. doi: 10.1007/s00217-018-3144-y. [DOI] [Google Scholar]
- 52.Barišić V., Kopjar M., Jozinović A., Flanjak I., Ačkar Đ., Miličević B., Šubarić D., Jokić S., Babić J. The chemistry behind chocolate production. Molecules. 2019;24:3163. doi: 10.3390/molecules24173163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y., Feng Y., Zhu S., Luo C., Ma J., Zhong F. The effect of alkalization on the bioactive and flavor related components in commercial cocoa powder. J. Food Compos. Anal. 2012;25:17–23. doi: 10.1016/j.jfca.2011.04.010. [DOI] [Google Scholar]
- 54.Valverde García D., Pérez Esteve É., Barat Baviera J.M. Changes in cocoa properties induced by the alkalization process: A review. Compr. Rev. Food Sci. Food Saf. 2020;19:2200–2221. doi: 10.1111/1541-4337.12581. [DOI] [PubMed] [Google Scholar]
- 55.Sulistyowati M., Misnawi J. Effects of alkali concentration and conching temperature on antioxidant activity and physical properties of chocolate. Int. Food Res. J. 2008;15:297–304. [Google Scholar]
- 56.Afoakwa E.O., Paterson A., Fowler M., Ryan A. Flavor formation and character in cocoa and chocolate: A critical review. Crit. Rev. Food Sci. Nutr. 2008;48:840–857. doi: 10.1080/10408390701719272. [DOI] [PubMed] [Google Scholar]
- 57.Di Mattia C.D., Martuscelli M., Sacchetti G., Beheydt B., Mastrocola D., Pittia P. Effect of different conching processes on procyanidins content and antioxidant properties of chocolate. Food Res. Int. 2014;63:367–372. doi: 10.1016/j.foodres.2014.04.009. [DOI] [Google Scholar]
- 58.Bordin Schumacher A.B., Brandelli A., Schumacher E.W., Macedo F.C., Pieta L., Klug T.V. Development and evaluation of a laboratory-scale conch for chocolate production. Int. J. Food Sci. Technol. 2009;44:606–622. doi: 10.1111/j.1365-2621.2008.01877.x. [DOI] [Google Scholar]
- 59.Albak F., Tekin A.R. Variation of total aroma and polyphenol content of dark chocolate during three phase of conching. J. Food Sci. Technol. 2016;53:848–855. doi: 10.1007/s13197-015-2036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gültekin-Ozgüven M., Berktas I., Ozçelik B. Influence of processing conditions on procyanidin profiles and antioxidant capacity of chocolates: Optimization of dark chocolate manufacturing by response surface methodology. LWT Food Sci. Technol. 2016;66:252–259. doi: 10.1016/j.lwt.2015.10.047. [DOI] [Google Scholar]
- 61.De Vuyst L., Weckx S. The cocoa bean fermentation process: From ecosystem analysis to starter culture development. J. Appl. Microbiol. 2016;121:5–17. doi: 10.1111/jam.13045. [DOI] [PubMed] [Google Scholar]
- 62.Wollgast J., Anklam E. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000;33:423–447. doi: 10.1016/S0963-9969(00)00068-5. [DOI] [Google Scholar]
- 63.Afoakwa E.O. Chocolate Science and Technology. 1st ed. Wiley-Blackwell Publishers; Oxford, UK: 2010. pp. 3–82. [Google Scholar]
- 64.Thompson S.S., Miller K.B., Lopez A., Camu N. Cocoa and coffee. In: Doyle M.P., Beuchat R.L., editors. Food Microbiology: Fundamentals and Frontiers. 4th ed. ASM Press; Washington, DC, USA: 2013. pp. 881–889. [Google Scholar]
- 65.Prabhakaran Nair K.P. Cocoa (Theobroma cacao L.) In: Prabhakaran Nair K.P., editor. The Agronomy and Economy of Important Tree Crops of the Developing World. Elsevier; Amsterdam, The Netherlands: 2010. pp. 131–180. Chapter 5. [DOI] [Google Scholar]
- 66.Dzelagha B.F., Ngwa N.M., Nde Bup D. A review of cocoa drying technologies and the effect on bean quality parameters. Int. J. Food Sci. 2020;2020:8830127. doi: 10.1155/2020/8830127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ackah E., Dompey E. Effects of fermentation and drying durations on the quality of cocoa (Theobroma cacao L.) beans during the rainy season in the Juaboso District of the Western-North Region, Ghana. Bull. Natl. Res. Cent. 2021;45:175. doi: 10.1186/s42269-021-00634-7. [DOI] [Google Scholar]
- 68.Zahouli G.I.B., Guehi S.T., Fae A.M., Nemlin J.G. Effect of drying methods on the chemical quality traits of cocoa raw material. Adv. J. Food Sci. Technol. 2010;2:184–190. [Google Scholar]
- 69.Saltini R., Akkerman R., Frosc S. Optimizing chocolate production through traceability: A review of the influence of farming practices on cocoa bean quality. Food Control. 2013;29:167–187. doi: 10.1016/j.foodcont.2012.05.054. [DOI] [Google Scholar]
- 70.Bharath S., Bowen-O’Connor C. Assessing Drying Rates of Cacao Beans Using Small Samples. Cocoa Research Unit; St. Augustine, Trinidad and Tobago: 2008. [(accessed on 20 July 2022)]. pp. 52–58. U.W.I. Annual Report. Available online: https://www.worldcocoafoundation.org/wp-content/uploads/files_mf/bharath2008.pdf. [Google Scholar]
- 71.Kamphuis H.J. Production and quality standards of cocoa mass, cocoa butter and cocoa powder. In: Beckett S.T., editor. Industrial Chocolate Manufacture and Use. 4th ed. John Wiley & Sons; Chichester, UK: 2009. pp. 121–139. [DOI] [Google Scholar]
- 72.Moser A. Alkalizing cocoa and chocolate. Manuf. Confect. 2015;95:31–38. [Google Scholar]
- 73.Sommer J. Developments in cocoa bean processing. Manuf. Confect. 2009;89:99. [Google Scholar]
- 74.Sioriki E., Tuenter E., Van de Walle D., Lemarcq V., Cazin C.S., Nolan S.P., Pieters L., Dewettinck K. The effect of cocoa alkalization on the non-volatile and volatile mood-enhancing compounds. Food Chem. 2022;381:132082. doi: 10.1016/j.foodchem.2022.132082. [DOI] [PubMed] [Google Scholar]
- 75.Quelal-Vásconez M.A., Lerma-García M.J., Pérez-Esteve É., Arnau-Bonachera A., Barat J.M., Talens P. Changes in methylxanthines and flavanols during cocoa powder processing and their quantification by near-infrared spectroscopy. LWT Food Sci. Technol. 2020;117:108598. doi: 10.1016/j.lwt.2019.108598. [DOI] [Google Scholar]
- 76.Dyer B. Alkalized cocoa powders; Proceedings of the 57th PMCA Production Conference; Hershey, PA, USA. 28–30 April 2003. [Google Scholar]
- 77.Ziegleder G. Flavour development in cocoa and chocolate. In: Beckett S.T., Fowler M.S., Ziegler G.R., editors. Industrial Chocolate Manufacture and Use. 5th ed. Wiley-Blackwell; Hoboken, NJ, USA: 2017. pp. 185–215. [Google Scholar]
- 78.Misnawi J., Febrianto N., Ariza B.T.S. Roasting equipment for cocoa processing. In: Hii C.L., Borém F.M., editors. Drying and Roasting of Cocoa and Coffee. CRC Press—Taylor & Francis Group; Boca Raton, FL, USA: 2020. pp. 47–62. [Google Scholar]
- 79.Krysiak W., Adamski R., Żyżelewicz D. Factors affecting the colour of roasted cocoa bean. J. Food Qual. 2013;36:21–31. doi: 10.1111/jfq.12009. [DOI] [Google Scholar]
- 80.Beckett S.T. Traditional chocolate making. In: Beckett S.T., Fowler M.S., Ziegler G.R., editors. Industrial Chocolate Manufacture and Use. 5th ed. Wiley-Blackwell; Hoboken, NJ, USA: 2017. pp. 1–9. [Google Scholar]
- 81.Kothe L., Zimmermann B.F., Galensa R. Temperature influences epimerization and composition of flavanol monomers, dimers and trimers during cocoa bean roasting. Food Chem. 2013;141:3656–3663. doi: 10.1016/j.foodchem.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 82.Ioannone F., Di Mattia C., De Gregorio M., Sergi M., Serafini M., Sacchetti G. Flavanols, proanthocyanidins and antioxidant activity changes during cocoa (Theobroma cacao L.) roasting as affected by temperature and time of processing. Food Chem. 2015;174:256–262. doi: 10.1016/j.foodchem.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 83.Stadler R.H., Hughes G., Guillaume-Gentil O. Safety of food and beverages: Coffee, tea and herbals, cocoa and derived products. In: Motarjemi Y., Moy G.G., Todd E.C.D., editors. Encyclopedia of Food Safety. Volume 3. Elsevier; Amsterdam, The Netherlands: 2014. pp. 371–383. [DOI] [Google Scholar]
- 84.Biehl B., Ziegleder G. Cocoa | Production, products, and use. In: Caballero B., editor. Encyclopedia of Food Sciences and Nutrition. 2nd ed. Academic Press; Cambridge, MA, USA: 2003. pp. 1448–1463. [Google Scholar]
- 85.Velthuis T. The nib-grinding process. Manuf. Confect. 2009;89:95. [Google Scholar]
- 86.Anoraga S.B., Wijanarti S., Sabarisman I. Effect of extraction time and pressing temperature on characteristic of cocoa powder quality. IOP Conf. Ser. Earth Environ. Sci. 2019;355:012050. doi: 10.1088/1755-1315/355/1/012050. [DOI] [Google Scholar]
- 87.Gutiérrez T.J. State-of-the-art chocolate manufacture: A review. Compr. Rev. Food Sci. Food Saf. 2017;16:1313–1344. doi: 10.1111/1541-4337.12301. [DOI] [PubMed] [Google Scholar]
- 88.Owusu M., Petersen M.A., Heimdal H. Effect of fermentation method roasting and conching conditions on the aroma volatiles of dark chocolate. J. Food Process. Preserv. 2012;36:446–456. doi: 10.1111/j.1745-4549.2011.00602.x. [DOI] [Google Scholar]
- 89.Toker O.S., Palabiyik I., Konar N. Chocolate quality and conching. Trends Food Sci. Technol. 2019;91:446–453. doi: 10.1016/j.tifs.2019.07.047. [DOI] [Google Scholar]
- 90.Urbańska B., Kowalska H., Szulc K., Ziarno M., Pochitskaya I., Kowalska J. Comparison of the effects of conching parameters on the contents of three dominant flavan3-ols, rheological properties and sensory quality in chocolate milk mass based on liquor from unroasted cocoa beans. Molecules. 2021;26:2502. doi: 10.3390/molecules26092502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Konar N. Influence of conching temperature and some bulk sweeteners on physical and rheological properties of prebiotic milk chocolate including containing inulin. Eur. Food Res. Technol. 2013;23:135–143. doi: 10.1007/s00217-012-1873-x. [DOI] [Google Scholar]
- 92.Owusu M., Petersen M.A., Heimdal H. Relationship of sensory and instrumental aroma measurements of dark chocolate as influenced by fermentation method, roasting and conching conditions. J. Food Sci. Technol. 2013;50:909–917. doi: 10.1007/s13197-011-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Engeseth N.J., Pangan M.F.A. Current context on chocolate flavor development—A review. Curr. Opin. Food Sci. 2018;21:84–91. doi: 10.1016/j.cofs.2018.07.002. [DOI] [Google Scholar]
- 94.Tiefenbacher K.F. Adjuncts—Filling creams, inclusions, cacao and chocolate. In: Tiefenbacher K.F., editor. Wafer and Waffle. Academic Press; Cambridge, MA, USA: 2017. pp. 313–404. Chapter 5. [Google Scholar]
- 95.Caligiani A., Marseglia A., Palla G. Cocoa: Production, chemistry, and use. In: Caballero B., Finglas P.M., Toldrá F., editors. The Encyclopedia of Food and Health. Volume 5. Academic Press; Cambridge, MA, USA: 2016. pp. 185–190. [DOI] [Google Scholar]
- 96.Martín M.A., Goya L., Ramos S. Anti-diabetic actions of cocoa flavonoids. Mol. Nutr. Food Res. 2016;60:1756–1769. doi: 10.1002/mnfr.201500961. [DOI] [PubMed] [Google Scholar]
- 97.Granado-Serrano A.B., Martín M.A., Izquierdo-Pulido M., Goya L., Bravo L., Ramos S. Molecular mechanisms of (−)-epicatechin and chlorogenic acid on the regulation of the apoptotic and survival/proliferation pathways in a human hepatoma cell line (HepG2) J. Agric. Food Chem. 2007;55:2020–2027. doi: 10.1021/jf062556x. [DOI] [PubMed] [Google Scholar]
- 98.Granado-Serrano A.B., Martín M.A., Goya L., Bravo L., Ramos S. Time-course regulation of survival pathways by epicathechin on HepG2. J. Nutr. Biochem. 2009;20:115–124. doi: 10.1016/j.jnutbio.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 99.Martín M.A., Granado-Serrano A.B., Ramos S., Izquierdo-Pulido M., Bravo L., Goya L. Cocoa flavonoids up-regulate antioxidant enzymes activity via ERK1/2 pathway to protect against oxidative stress-induced apoptosis in HepG2 cells. J. Nutr. Biochem. 2010;21:196–205. doi: 10.1016/j.jnutbio.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 100.Granado-Serrano A.B., Martín M.A., Haegeman G., Goya L., Bravo L., Ramos S. Epicatechin induces NF-κB, activator protein-1 (AP-1) and nuclear transcription factor erythroid 2p45-related (Nrf2) via phosphatidylinositol-3-kiinase/protein kinase B (PI3K/AKT) and extracellular regulated kinase (ERK) signalling in HepG2 cells. Brit. J. Nutr. 2010;103:168–179. doi: 10.1017/S0007114509991747. [DOI] [PubMed] [Google Scholar]
- 101.Rodríguez-Ramiro I., Ramos S., Bravo L., Goya L., Martín M.A. Procyanidin B2 and a cocoa polyphenolic extract inhibit acrylamide-induced apoptosis in human Caco-2 cells by preventing oxidative stress and activation of JNK pathway. J. Nutr. Biochem. 2011;22:1186–1194. doi: 10.1016/j.jnutbio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 102.Rodríguez-Ramiro I., Ramos S., Bravo L., Goya L., Martín M.A. Procyanidin B2 induces Nrf2 translocation and glutathione-S-transferase P1 expression and via ERKs and p38-MAPK pathways and protect human colonic cells against oxidative stress. Eur. J. Nutr. 2012;51:881–892. doi: 10.1007/s00394-011-0269-1. [DOI] [PubMed] [Google Scholar]
- 103.Cordero-Herrera I., Martín M.A., Bravo L., Goya L., Ramos S. Cocoa flavonoids improve insulin signalling and repress glucose production via AKT and AMPK in HepG2 cells. Mol. Nutr. Food Res. 2013;57:974–985. doi: 10.1002/mnfr.201200500. [DOI] [PubMed] [Google Scholar]
- 104.Cordero-Herrera I., Martín M.A., Goya L., Ramos S. Cocoa flavonoids protect hepatic cells against high glucose-induced oxidative stress: Relevance of MAPKs. Mol. Nutr. Food Res. 2015;59:597–609. doi: 10.1002/mnfr.201400492. [DOI] [PubMed] [Google Scholar]
- 105.Fernández-Millán E., Ramos S., Alvarez C., Bravo L., Goya L., Martín M.A. Microbial phenolic metabolites improve glucose-stimulated insulin secretion and protect pancreatic beta cells against oxidative stress via ERKs and PKC pathways. Food Chem. Toxicol. 2014;66:245–253. doi: 10.1016/j.fct.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 106.Álvarez-Cilleros D., Martín M.A., Goya L., Ramos S. (−)-Epicatechin and the colonic metabolite 3,4-dihydroxyphenylacetic acid protect renal proximal tubular cell against high glucose-induced oxidative stress by modulating NOX-4/SIRT-1 signalling. J. Funct. Foods. 2018;46:19–28. doi: 10.1016/j.jff.2018.04.051. [DOI] [Google Scholar]
- 107.Zhihao Q., Ailing L., Penghui L., Changwei L., Wenjun X., Jianan H., Zhonghua L., Sheng Z. Advances in physiological functions and mechanisms of (−)-epicatechin. Crit. Rev. Food Sci. Nutr. 2021;61:211–233. doi: 10.1080/10408398.2020.1723057. [DOI] [PubMed] [Google Scholar]
- 108.Gao M., Peng X., Tang J., Deng J., Wang F., Zhang Y., Zhao P., Kan H., Liu Y. Anti-Inflammatory Effects of Camellia fascicularis Polyphenols via Attenuation of NF-κB and MAPK Pathways in LPS-Induced THP-1 Macrophages. J. Inflamm. Res. 2022;15:851–864. doi: 10.2147/JIR.S349981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim J.K., Yang H.J., Go Y. Quercus acuta Thunb. Suppresses LPS-Induced Neuroinflammation in BV2 Microglial Cells via Regulating MAPK/NF-κB and Nrf2/HO-1 Pathway. Antioxidants. 2022;11:1851. doi: 10.3390/antiox11101851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang P., Hong J., Mi J., Sun B., Zhang J., Li C., Yang W. Polyphenols extracted from Enteromorpha clathrata alleviates inflammation in lipopolysaccharide-induced RAW 264.7 cells by inhibiting the MAPKs/NF-κB signaling pathways. J. Ethnopharmacol. 2022;286:114897. doi: 10.1016/j.jep.2021.114897. [DOI] [PubMed] [Google Scholar]
- 111.Khan A., Khan S.U., Khan A., Shal B., Rehman S.U., Rehman S.U., Htar T.T., Khan S., Anwar S., Alafnan A., et al. Anti-inflammatory and anti-rheumatic potential of selective plant compounds by targeting TLR-4/AP-1 signaling: A comprehensive molecular docking and simulation approaches. Molecules. 2022;27:4319. doi: 10.3390/molecules27134319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luo Y., Lu J., Wang Z., Wang L., Wu G., Guo Y., Dong Z. Small ubiquitin-related modifier (SUMO)ylation of SIRT1 mediates (−)-epicatechin inhibited- differentiation of cardiac fibroblasts into myofibroblasts. Pharm. Biol. 2022;60:1762–1770. doi: 10.1080/13880209.2022.2101672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Behl T., Upadhyay T., Singh S., Chigurupati S., Alsubayiel A.M., Mani V., Vargas-De-La-Cruz C., Uivarosan D., Bustea C., Sava C., et al. Polyphenols targeting MAPK mediated oxidative stress and inflammation in rheumatoid arthritis. Molecules. 2021;26:6570. doi: 10.3390/molecules26216570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cháirez-Ramírez M.H., de la Cruz-López K.G., García-Carrancá A. Polyphenols as antitumor agents targeting key players in cancer-driving signaling pathways. Front. Pharmacol. 2021;12:710304. doi: 10.3389/fphar.2021.710304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Anjum J., Mitra S., Das R., Alam R., Mojumder A., Emran T.B., Islam F., Rauf A., Hossain J., Aljohani A.S.M., et al. A renewed concept on the MAPK signaling pathway in cancers: Polyphenols as a choice of therapeutics. Pharmacol. Res. 2022;184:106398. doi: 10.1016/j.phrs.2022.106398. [DOI] [PubMed] [Google Scholar]
- 116.Behl T., Mehta K., Sehgal A., Singh S., Sharma N., Ahmadi A., Arora S., Bungau S. Exploring the role of polyphenols in rheumatoid arthritis. Crit. Rev. Food Sci. Nutr. 2022;62:5372–5393. doi: 10.1080/10408398.2021.1924613. [DOI] [PubMed] [Google Scholar]
- 117.Hazafa A., Iqbal M.O., Javaid U., Tareen M.B.K., Amna D., Ramzan A., Piracha S., Naeem M. Inhibitory effect of polyphenols (phenolic acids, lignans and stilbenes) on cancer by regulating signal transduction pathways: A review. Clin. Transl. Oncol. 2022;24:432–445. doi: 10.1007/s12094-021-02709-3. [DOI] [PubMed] [Google Scholar]
- 118.Zheng Z., Zhang L., Hou X. Potential roles and molecular mechanisms of phytochemicals against cancer. Food Funct. 2022;13:9208–9225. doi: 10.1039/D2FO01663J. [DOI] [PubMed] [Google Scholar]
- 119.Bernatoniene J., Kopustinskiene D.M. The role of catechins in cellular responses to oxidative stress. Molecules. 2018;23:965. doi: 10.3390/molecules23040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ruiz-Iglesias P., Massot-Cladera M., Rodríguez-Lagunas M.J., Franch A., Camps-Bossacoma M., Pérez-Cano F.J., Castell M. Protective effect of a cocoa-enriched diet on oxidative stress induced by intensive acute exercise in rats. Antioxidants. 2022;11:753. doi: 10.3390/antiox11040753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smith R.E. The effects of dietary supplements that overactivate the Nrf2/ARE system. Curr. Med. Chem. 2020;27:2077–2094. doi: 10.2174/0929867326666190517113533. [DOI] [PubMed] [Google Scholar]
- 122.Bahia P., Rattray M., Williams R. Dietary flavonoid (−) epicatechin stimulates phosphatidylinositol 3-kinase-dependent anti-oxidant response element activity and up-regulates glutathione in cortical astrocytes. J. Neurochem. 2008;106:2194–2204. doi: 10.1111/j.1471-4159.2008.05542.x. [DOI] [PubMed] [Google Scholar]
- 123.Cheng Y.T., Wu C.H., Ho C.Y., Yen G.C. Catechin protects against ketoprofen-induced oxidative damage of the gastric mucosa by up-regulating Nrf2 in vitro and in vivo. J. Nutr. Biochem. 2013;24:475–483. doi: 10.1016/j.jnutbio.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 124.Nichols M., Zhang J., Polster B.M., Elustondo P.A., Thirumaran A., Pavlov E.V., Robertson G.S. Synergistic neuroprotection by epicatechin and quercetin: Activation of convergent mitochondrial signaling pathways. Neuroscience. 2015;308:75–94. doi: 10.1016/j.neuroscience.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 125.Ramírez-Sánchez I., Rodríguez A., Ulloa A.M., Ceballos G., Villarreal F. (−)-Epicatechin-induced recovery of mitochondria from simulated diabetes: Potential role of endothelial nitric oxide synthase. Diabetes Vasc. Dis. Res. 2016;13:201–210. doi: 10.1177/1479164115620982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rowley T.J., IV, Bitner B.F., Ray J.D., Lathen D.R., Smithson A.T., Dallon B.W., Plowman C.J., Bikman B.T., Hansen J.M., Dorenkott M.R., et al. Monomeric cocoa catechins enhance β-cell function by increasing mitochondrial respiration. J. Nutr. Biochem. 2017;49:30–41. doi: 10.1016/j.jnutbio.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 127.Sapian S., Taib I.S., Latip J., Katas H., Chin K.Y., Nor N.A.M., Jubaidi F.F., Budin S.B. Therapeutic Approach of Flavonoid in Ameliorating Diabetic Cardiomyopathy by Targeting Mitochondrial-Induced Oxidative Stress. Int. J. Mol. Sci. 2021;22:11616. doi: 10.3390/ijms222111616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hamsalakshmi A.M.A., Mahalakshmi A.M., Joghee S., Chidambaram S.B. Therapeutic benefits of flavonoids against neuroinflammation: A systematic review. Inflammopharmacology. 2022;30:111–136. doi: 10.1007/s10787-021-00895-8. [DOI] [PubMed] [Google Scholar]
- 129.Clifford T., Acton J.P., Cocksedge S.P., Bowden Davies K.A., Bailey S.J. The effect of dietary phytochemicals on nuclear factor erythroid 2-related factor 2 (Nrf2) activation: A systematic review of human intervention trials. Mol. Biol. Rep. 2021;48:1745–1761. doi: 10.1007/s11033-020-06041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bustamante-Pozo M., Haro M., Garcia R., Carson N., Ceballos G., Villarreal F. Epicatechin ameliorates cardiac fibrosis in a female rat model of pre-heart failure with preserved ejection fraction. J. Med. Food. 2022;25:836–844. doi: 10.1089/jmf.2021.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Daussin F.N., Cuillerier A., Touron J., Bensaid S., Melo B., Rewashdy A.A., Vasam G., Menzies K.J., Harper M.E., Heyman E., et al. Dietary cocoa flavanols enhance mitochondrial function in skeletal muscle and modify whole-body metabolism in healthy mice. Nutrients. 2021;13:3466. doi: 10.3390/nu13103466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.García-Díez E., López-Oliva M.E., Caro-Vadillo A., Pérez-Vizcaíno F., Pérez-Jiménez J., Ramos S., Martín M.A. Supplementation with a cocoa–carob blend, alone or in combination with metformin, attenuates diabetic cardiomyopathy, cardiac oxidative stress and inflammation in zucker diabetic rats. Antioxidants. 2022;11:432. doi: 10.3390/antiox11020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.García-Merino J.A., de Lucas B., Herrera-Rocha K., Moreno-Pérez D., Montalvo-Lominchar M.G., Fernández-Romero A., Santiago C., Pérez-Ruiz M., Larrosa M. Flavanol-rich cocoa supplementation inhibits mitochondrial biogenesis triggered by exercise. Antioxidants. 2022;11:1522. doi: 10.3390/antiox11081522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Muralidhara K.G. Dietary Supplements as Cognitive Enhancers: The Role of Flavonoid-Rich Foods and their Relevance in Age-Related Neurodegeneration. In: Watson R.R., Preedy V.R., editors. Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease. Academic Press; Cambridge, MA, USA: 2015. pp. 281–290. [Google Scholar]
- 135.Vauzour D., Camprubi-Robles M., Miquel-Kergoat S., Andres-Lacueva C., Bánáti D., Barberger-Gateau P., Bowman G.L., Caberlotto L., Clarke R., Hogervorst E., et al. Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res. Rev. 2017;35:222–240. doi: 10.1016/j.arr.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 136.Dominguez L.J., Barbagallo M. Nutritional prevention of cognitive decline and dementia. Acta Biomed. 2018;89:276–290. doi: 10.23750/abm.v89i2.7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Socci V., Tempesta D., Desideri G., De Gennaro L., Ferrara M. Enhancing human cognition with cocoa flavonoids. Front. Nutr. 2017;4:19. doi: 10.3389/fnut.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Haskell-Ramsay C.F., Schmitt J., Actis-Goretta L. The impact of epicatechin on human cognition: The role of cerebral blood flow. Nutrients. 2018;10:986. doi: 10.3390/nu10080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Abd El Mohsen M.M., Kuhnle G., Rechner A.R., Schroeter H., Rose S., Jenner P., Rice-Evans C.A. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic. Biol. Med. 2002;33:1693–1702. doi: 10.1016/S0891-5849(02)01137-1. [DOI] [PubMed] [Google Scholar]
- 140.Francis S.T., Head K., Morris P.G., Macdonald I.A. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 2006;47((Suppl. S2)):S215–S220. doi: 10.1097/00005344-200606001-00018. [DOI] [PubMed] [Google Scholar]
- 141.Kelleher R.J., III, Govindarajan A., Jung H.Y., Kang H., Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/S0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 142.Schroeter H., Bahia P., Spencer J.P., Sheppard O., Rattray M., Cadenas E., Rice-Evans C., Williams R.J. (−)Epicatechin stimulates ERK-dependent cyclic AMP response element activity and upregulates GluR2 in cortical neurons. J. Neurochem. 2007;101:1596–1606. doi: 10.1111/j.1471-4159.2006.04434.x. [DOI] [PubMed] [Google Scholar]
- 143.Desideri G., Kwik-Uribe C., Grassi D., Necozione S., Ghiadoni L., Mastroiacovo D., Ferri C. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: The Cocoa, Cognition, and Aging (CoCoA) study. Hypertension. 2012;60:794–801. doi: 10.1161/HYPERTENSIONAHA.112.193060. [DOI] [PubMed] [Google Scholar]
- 144.Pase M.P., Scholey A.B., Pipingas A., Kras M., Nolidin K., Gibbs A., Stough C. Cocoa polyphenols enhance positive mood states but not cognitive performance: A randomized, placebo-controlled trial. J. Psychopharmacol. 2013;27:451–458. doi: 10.1177/0269881112473791. [DOI] [PubMed] [Google Scholar]
- 145.Lamport D.J., Pal D., Moutsiana C., Field D.T., Williams C.M., Spencer J.P., Butler L.T. The effect of flavanol-rich cocoa on cerebral perfusion in healthy older adults during conscious resting state: A placebo controlled, crossover, acute trial. J. Psychopharmacol. 2015;232:3227–3234. doi: 10.1007/s00213-015-3972-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ammar A., Trabelsi K., Müller P., Bouaziz B., Boukhris O., Glenn J.M., Bott N.T., Driss T., Chtourou H., Müller N., et al. The effect of (poly)phenol-rich interventions on cognitive functions and neuroprotective measures in healthy aging adults: A systematic review and meta-analysis. J. Clin. Med. 2020;9:835. doi: 10.3390/jcm9030835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Barrera-Reyes P.K., De Lara J.C.-F., González-Soto M., Tejero M. Effects of cocoa-derived polyphenols on cognitive function in humans. Systematic review and analysis of methodological aspects. Plant Foods Hum. Nutr. 2020;75:1–11. doi: 10.1007/s11130-019-00779-x. [DOI] [PubMed] [Google Scholar]
- 148.Di Meo F., Valentino A., Petillo O., Peluso G., Filosa S., Crispi S. Bioactive polyphenols and neuromodulation: Molecular mechanisms in neurodegeneration. Int. J. Mol. Sci. 2020;21:2564. doi: 10.3390/ijms21072564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Flanagan E., Lamport D., Brennan L., Burnet P., Calabrese V., Cunnane S.C., de Wilde M.C., Dye L., Farrimond J.A., Emerson Lombardo N., et al. Nutrition and the ageing brain: Moving towards clinical applications. Ageing Res. Rev. 2020;62:101079. doi: 10.1016/j.arr.2020.101079. [DOI] [PubMed] [Google Scholar]
- 150.Martín M.A., Goya L., de Pascual-Teresa S. Effect of cocoa and cocoa products on cognitive performance in young adults. Nutrients. 2020;12:3691. doi: 10.3390/nu12123691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vinciguerra F., Graziano M., Hagnäs M., Frittitta L., Tumminia A. Influence of the Mediterranean and ketogenic diets on cognitive status and decline: A narrative review. Nutrients. 2020;12:1019. doi: 10.3390/nu12041019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kang J., Wang Z., Oteiza P.I. (−)-Epicatechin mitigates high fat diet-induced neuroinflammation and altered behavior in mice. Food Funct. 2020;11:5065–5076. doi: 10.1039/D0FO00486C. [DOI] [PubMed] [Google Scholar]
- 153.Gratton G., Weaver S.R., Burley C.V., Low K.A., Maclin E.L., Johns P.W., Pham Q.S., Lucas S.J.E., Fabiani M., Rendeiro C. Dietary flavanols improve cerebral cortical oxygenation and cognition in healthy adults. Sci. Rep. 2020;10:19409. doi: 10.1038/s41598-020-76160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Baynham R., Veldhuijzen van Zanten J.J.C.S., Johns P.W., Pham Q.S., Rendeiro C. Cocoa flavanols improve vascular responses to acute mental stress in young healthy adults. Nutrients. 2021;13:1103. doi: 10.3390/nu13041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Navarrete-Yañez V., Garate-Carrillo A., Ayala M., Rodriguez-Castañeda A., Mendoza-Lorenzo P., Ceballos G., Ordoñez-Razo R., Dugar S., Schreiner G., Villarreal F., et al. Stimulatory effects of (−)-epicatechin and its enantiomer (+)-epicatechin on mouse frontal cortex neurogenesis markers and short-term memory: Proof of concept. Food Funct. 2021;12:3504. doi: 10.1039/D0FO03084H. [DOI] [PubMed] [Google Scholar]
- 156.García-Cordero J., Pino A., Cuevas C., Puertas-Martín V., San Román R., de Pascual-Teresa S. Neurocognitive effects of cocoa and red-berries consumption in healthy adults. Nutrients. 2022;14:1. doi: 10.3390/nu14010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Caruso G., Torrisi S.A., Mogavero M.P., Currenti W., Castellano S., Godos J., Ferri R., Galvano F., Leggio G.M., Grosso G., et al. Polyphenols and neuroprotection: Therapeutic implications for cognitive decline. Pharmacol. Ther. 2022;232:108013. doi: 10.1016/j.pharmthera.2021.108013. [DOI] [PubMed] [Google Scholar]
- 158.Lalonde R., Strazielle C. Cocoa flavanols and the aging brain. Curr. Aging Sci. 2022;15 doi: 10.2174/1874609815666220819145845. [DOI] [PubMed] [Google Scholar]
- 159.Suominen M.H., Laaksonen M.M.L., Salmenius-Suominena H., Kautiainen H., Hongisto S.M., Tuukkanen K., Jyväkorpi S.K., Pitkälä K.H. The short-term effect of dark chocolate flavanols on cognition in older adults: A randomized controlled trial (FlaSeCo) Exp. Gerontol. 2020;136:110933. doi: 10.1016/j.exger.2020.110933. [DOI] [PubMed] [Google Scholar]
- 160.Baker L.D., Manson J.E., Rapp S.R., Sesso H.D., Gaussoin S.A., Shumaker S.A., Espeland M.A. Effects of cocoa extract and a multivitamin on cognitive function: A randomized clinical trial. Alzheimer Dement. 2022:1–12. doi: 10.1002/alz.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shishtar E., Rogers G.T., Blumberg J.B., Au R., Jacques P.F. Long-term dietary flavonoid intake and change in cognitive function in the Framingham Offspring Cohort. Public Health Nutr. 2020;23:1576–1588. doi: 10.1017/S136898001900394X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gardener S.L., Rainey-Smith S.R., Weinborn M., Bondonno C.P., Martins R.N. Intake of products containing anthocyanins, flavanols, and flavanones, and cognitive function: A narrative review. Front. Aging Neurosci. 2021;13:640381. doi: 10.3389/fnagi.2021.640381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lamport D.J., Williams C.M. Polyphenols and cognition in humans: An overview of current evidence from recent systematic reviews and meta-analyses. Brain Plast. 2021;6:139–153. doi: 10.3233/BPL-200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rodrigo-Gonzalo M.J., González-Manzano S., Mendez-Sánchez R., Santos-Buelga C., Recio-Rodríguez J.I. Effect of polyphenolic complements on cognitive function in the elderly: A systematic review. Antioxidants. 2022;11:1549. doi: 10.3390/antiox11081549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zeli C., Lombardo M., Storz M.A., Ottaviani M., Rizzo G. Chocolate and cocoa-derived biomolecules for brain cognition during ageing. Antioxidants. 2022;11:1353. doi: 10.3390/antiox11071353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.European Food Safety Authority Scientific Opinion on the substantiation of health claims related to cocoa flavanols and protection of lipids from oxidative damage and maintenance of normal blood pressure. EFSA J. 2010;8:1792. [Google Scholar]
- 167.European Food Safety Authority Scientific opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation. EFSA J. 2012;10:2809. [Google Scholar]
- 168.Sun Y., Zimmermann D., De Castro C.A., Actis-Goretta L. Dose-response relationship between cocoa flavanols and human endothelial function: A systematic review and meta-analysis of randomized trials. Food Funct. 2019;10:6322. doi: 10.1039/C9FO01747J. [DOI] [PubMed] [Google Scholar]
- 169.Kris-Etherton P.M., Keen C.L. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr. Opin. Lipidol. 2002;13:41–49. doi: 10.1097/00041433-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 170.Heiss C., Dejam A., Kleinbongard P., Schewe T., Sies H., Kelm M. Vascular effects of cocoa rich in flavan-3-ols. J. Am. Med. Assoc. 2003;290:1030–1031. doi: 10.1001/jama.290.8.1030. [DOI] [PubMed] [Google Scholar]
- 171.Fisher N.D., Hughes M., Gerhard-Herman M., Hollenberg N.K. Flavanol rich cocoa induces nitric oxide-dependent vasodilation in healthy humans. J. Hypertens. 2003;1:2281–2286. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 172.Hollenberg N.K. Vascular action of cocoa flavanols in humans: The roots of the story. J. Cardiovasc. Pharmacol. 2006;47:S99–S102. doi: 10.1097/00005344-200606001-00002. [DOI] [PubMed] [Google Scholar]
- 173.Schroeter H., Heiss C., Balzer J., Kleinbongard P., Keen C.L., Hollenberg N.K., Sies H., Kwik-Uribe C., Schmitz H.H., Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA. 2006;3:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Steffen Y., Schewe T., Sies H. (−)-Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem. Biophys. Res. Commun. 2007;359:828–833. doi: 10.1016/j.bbrc.2007.05.200. [DOI] [PubMed] [Google Scholar]
- 175.Taubert D., Roesen R., Lehmann C., Jung N., Schömig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide. J. Am. Med. Assoc. 2007;298:49–60. doi: 10.1001/jama.298.1.49. [DOI] [PubMed] [Google Scholar]
- 176.Balzer J., Rassaf T., Heiss C., Kleinbongard P., Lauer T., Merx M., Heussen N., Gross H.B., Keen C.L., Schroeter H., et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients. a double-masked, randomized, controlled trial. J. Am. Coll. Cardiol. 2008;51:2141–2149. doi: 10.1016/j.jacc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 177.Faridi Z., Njike V.Y., Dutta S., Ali A., Katz D.L. Acute dark chocolate and cocoa ingestion and endothelial function: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2008;8:58–63. doi: 10.1093/ajcn/88.1.58. [DOI] [PubMed] [Google Scholar]
- 178.Grassi D., Desideri G., Necozione S., Lippi C., Casale R., Properzi G., Blumberg J.B., Ferri C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high polyphenol dark chocolate. J. Nutr. 2008;38:1671–1676. doi: 10.1093/jn/138.9.1671. [DOI] [PubMed] [Google Scholar]
- 179.Hooper L., Kroon P.A., Rimm E.B., Cohn J.S., Harvey I., Le Cornu K.A., Ryder J.J., Hall W.L., Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 180.Ried K., Sullivan T.R., Fakler P., Frank O., Stocks N. Does chocolate reduce blood pressure? A meta-analysis. BMC Med. 2010;8:39. doi: 10.1186/1741-7015-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ried K., Sullivan T.R., Fakler P., Frank O., Stocks N.P. Effect of cocoa on blood pressure. Cochrane Database Syst. Rev. 2012;15:CD008893. doi: 10.1002/14651858.CD008893.pub3. [DOI] [PubMed] [Google Scholar]
- 182.de Pascual-Teresa S., Clifford M.N. Advances in polyphenol research: A journal of agricultural and food chemistry virtual issue. J. Agric. Food Chem. 2017;65:8093–8095. doi: 10.1021/acs.jafc.7b04055. [DOI] [PubMed] [Google Scholar]
- 183.Ludovici V., Barthelmes J., Nägele M.P., Enseleit F., Ferri C., Flammer A.J., Ruschitzka F., Sudano I. Cocoa, blood pressure, and vascular function. Front. Nutr. 2017;4:36. doi: 10.3389/fnut.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dugo L., Tripodo G., Santi L., Fanali C. Cocoa polyphenols: Chemistry, bioavailability and effects on cardiovascular performance. Curr. Med. Chem. 2018;25:4903–4917. doi: 10.2174/0929867323666160919094339. [DOI] [PubMed] [Google Scholar]
- 185.Mozaffarian D., Wu J.H.Y. Flavonoids, dairy foods, and cardiovascular and metabolic health. A review of emerging biologic pathways. Circ Res. 2018;122:369–384. doi: 10.1161/CIRCRESAHA.117.309008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ciumarnean L., Milaciu M.V., Runcan O., Vesa S.C., Rachis A.L., Negrean V., Perné M.-G., Donca V.I., Alexescu T.-G., Para I. The effects of flavonoids in cardiovascular diseases. Molecules. 2020;25:4320. doi: 10.3390/molecules25184320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ebaditabar M., Djafarian K., Saeidifard N., Shab-Bidar S. Effect of dark chocolate on flow-mediated dilatation: Systematic review, meta-analysis, and dose–response analysis of randomized controlled trials. Clin. Nutr. ESPEN. 2020;36:17–27. doi: 10.1016/j.clnesp.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 188.Martin M.A., Ramos S. Impact of cocoa flavanols on human health. Food Chem. Toxicol. 2021;151:112121. doi: 10.1016/j.fct.2021.112121. [DOI] [PubMed] [Google Scholar]
- 189.Azad B.J., Daneshzad E., Meysamie A.P., Koohdani F. Chronic and acute effects of cocoa products intake on arterial stiffness and platelet count and function: A systematic review and dose-response Meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2021;61:357–379. doi: 10.1080/10408398.2020.1733484. [DOI] [PubMed] [Google Scholar]
- 190.Tanghe A., Heyman E., Lespagnol E., Stautemas J., Celie B., Op ‘t Roodt J., Rietzschel E., Dias Soares D., Hermans N., Tuenter E., et al. Acute effects of cocoa flavanols on blood pressure and peripheral vascular reactivity in type 2 diabetes mellitus and essential hypertension. Nutrients. 2022;14:2692. doi: 10.3390/nu14132692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alañon M.E., Castle S.M., Serra G., Leveques A., Poquet L., Actis-Goretta L., Spencer J.P.E. Acute study of dose-dependent effects of (−)-epicatechin on vascular function in healthy male volunteers: A randomized controlled trial. Clin. Nutr. 2020;39:746–754. doi: 10.1016/j.clnu.2019.03.041. [DOI] [PubMed] [Google Scholar]
- 192.Gröne M., Sansone R., Höffken P., Horn P., Rodriguez-Mateos A., Schroeter H., Kelm M., Heiss C. Cocoa flavanols improve endothelial functional integrity in healthy young and elderly subjects. J. Agric. Food Chem. 2020;68:1871–1876. doi: 10.1021/acs.jafc.9b02251. [DOI] [PubMed] [Google Scholar]
- 193.Ottaviani J.I., Britten A., Lucarelli D., Luben R., Mulligan A.A., Lentjes M.A., Fong R., Gray N., Grace P.B., Mawson D.H., et al. Biomarker-estimated flavan-3-ol intake is associated with lower blood pressure in cross-sectional analysis in EPIC Norfolk. Sci. Rep. 2020;10:17964. doi: 10.1038/s41598-020-74863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Milenkovic D., Rodriguez-Mateos A., Lucosz M., Istas G., Declerck K., Sansone R., Deenen R., Köhrer K., Corral-Jara K.F., Altschmied J., et al. Flavanol consumption in healthy men preserves integrity of immunological-endothelial barrier cell functions: Nutri(epi)genomic analysis. Mol. Nutr. Food Res. 2022;66:2100991. doi: 10.1002/mnfr.202100991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sesso H.D., Manson J.E., Aragaki A.K., Rist P.M., Johnson L.G., Friedenberg G., Copeland T., Clar A., Mora S., Vinayaga Moorthy M., et al. Effect of cocoa flavanol supplementation for the prevention of cardiovascular disease events: The COcoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial. Am. J. Clin. Nutr. 2022;115:1490–1500. doi: 10.1093/ajcn/nqac055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jafarnejad S., Salek M., Clark C.C.T. Cocoa consumption and blood pressure in middle-aged and elderly subjects: A meta-analysis. Curr. Hypertens. Rep. 2020;22:1. doi: 10.1007/s11906-019-1005-0. [DOI] [PubMed] [Google Scholar]
- 197.Rashid M., Verhoeven A.J.M., Mulder M.T., Timman R., Ozcan B., van Beek-Nieuwland Y., Chow L.M., van de Laar R.J.J.M., Dik W.A., Sijbrands E.J.G., et al. The effect of monomeric and oligomeric FLAVAnols in patients with type 2 diabetes and microalbuminuria (FLAVA-trial): A double blind randomized controlled trial. Clin. Nutr. 2021;40:5587–5594. doi: 10.1016/j.clnu.2021.09.038. [DOI] [PubMed] [Google Scholar]
- 198.Cadoná F.C., Ferreira Dantas R., Haas de Mello G., Paes Silva F., Jr. Natural products targeting into cancer hallmarks: An update on caffeine, theobromine, and (+) catechin. Crit. Rev. Food Sci. Nutr. 2022;62:7222–7241. doi: 10.1080/10408398.2021.1913091. [DOI] [PubMed] [Google Scholar]
- 199.Monteiro J., Alves M.G., Oliveira P.F., Silva B.M. Pharmacological potential of methylxanthines: Retrospective analysis and future expectations. Crit. Rev. Food Sci. Nutr. 2019;59:2597–2625. doi: 10.1080/10408398.2018.1461607. [DOI] [PubMed] [Google Scholar]
- 200.Camps-Bossacoma M., Garcia-Aloy M., Saldaña-Ruiz S., Cambras T., Gonzalez-Domínguez R., Franch A., Perez-Cano F.J., Andres-Lacueva C., Castell M. Role of theobromine in cocoa’s metabolic properties in healthy rats. J. Agric. Food Chem. 2019;67:3605–3614. doi: 10.1021/acs.jafc.8b07248. [DOI] [PubMed] [Google Scholar]
- 201.Leehey D.J. Targeting inflammation in diabetic kidney disease: Is there a role for pentoxifylline? Kidney 360. 2020;1:292–299. doi: 10.34067/KID.0001252019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Aronsen L., Orvoll E., Lysaa R., Ravna A.W., Sager G. Modulation of high affinity ATP-dependent cyclic nucleotide transporters by specific and non-specific cyclic nucleotide phosphodiesterase inhibitors. Eur. J. Pharmacol. 2014;745:249–253. doi: 10.1016/j.ejphar.2014.10.051. [DOI] [PubMed] [Google Scholar]
- 203.Gil M., Skopinska-Rozewska E., Radomska D., Demkow U., Skurzak H., Rochowska M., Beuth J., Roszkowski K. Effect of purinergic receptor antagonists suramin and theobromine on tumor-induced angiogenesis in BALB/c mice. Folia Biol. 1993;39:63–68. [PubMed] [Google Scholar]
- 204.Barcz E., Sommer E., Sokolnicka I., Gawrychowski K., Roszkowska-Purska K., Janik P., Skopinska-Rozewska E. The influence of theobromine on angiogenic activity and proangiogenic cytokines production of human ovarian cancer cells. Oncol. Rep. 1998;5:517–537. doi: 10.3892/or.5.2.517. [DOI] [PubMed] [Google Scholar]
- 205.Sugimoto N., Miwa S., Hitomi Y., Nakamura H., Tsuchiya H., Yachie A. Theobromine, the primary methylxanthine found in theobroma cacao, prevents malignant glioblastoma proliferation by negatively regulating phosphodiesterase-4, extracellular signal-regulated kinase, Akt/mammalian target of rapamycin kinase, and nuclear factor-κB. Nutr. Cancer. 2014;66:419–423. doi: 10.1080/01635581.2013.877497. [DOI] [PubMed] [Google Scholar]
- 206.Jang Y.J., Koo H.J., Sohn E.H., Kang S.C., Rhee D.K., Pyo S. Theobromine inhibits differentiation of 3T3-L1 cells during the early stage of adipogenesis via AMPK and MAPK signaling pathways. Food Funct. 2015;6:2365–2374. doi: 10.1039/C5FO00397K. [DOI] [PubMed] [Google Scholar]
- 207.Papadimitriou A., Silva K.C., Peixoto E.B.M.I., Borges C.M., Lopes de Faria J.M., Lopes de Faria J.B. Theobromine increases NAD+/Sirt-1 activity and protects the kidney under diabetic conditions. Am. J. Physiol. Renal Physiol. 2015;308:F209–F225. doi: 10.1152/ajprenal.00252.2014. [DOI] [PubMed] [Google Scholar]
- 208.Cadonna F.C., Machado A.K., Azzolin V.F., Barbisan F., Dornelles E.B., Glanzner W., Goncalves P.B.D., Assmann C.E., Ribeiro E.E., da Cruz I.B.M. Guarana a caffeine-rich food increases oxaliplatin sensitivity of colorectal HT-29 cells by apoptosis pathway modulation. Anti-Cancer Agents Med. Chem. 2016;16:1055–1065. doi: 10.2174/1871520616666151217121138. [DOI] [PubMed] [Google Scholar]
- 209.Yoneda M., Sugimoto N., Katakura M., Matsuzaki K., Tanigami H., Yachi A., Ohno-Shosaku T., Shido O. Theobromine up-regulates cerebral brain-derived neurotrophic factor and facilitates motor learning in mice. J. Nutr. Biochem. 2017;39:110–116. doi: 10.1016/j.jnutbio.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 210.Mitani T., Watanabe S., Yoshioka Y., Katayama S., Nakamura S., Ashida H. Theobromine suppresses adipogenesis through enhancement of CCAAT enhancer-binding protein β degradation by adenosine receptor A1. BBA Mol. Cell Res. 2017;1864:2438–2448. doi: 10.1016/j.bbamcr.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 211.Shojaei-Zarghani S., Khosroushahi A.Y., Rafraf M. Oncopreventive effects of theanine and theobromine on dimethylhydrazine-induced colon cancer model. Biomed. Pharmacother. 2021;134:111140. doi: 10.1016/j.biopha.2020.111140. [DOI] [PubMed] [Google Scholar]
- 212.Tanaka E., Mitani T., Nakashima M., Yonemoto E., Fujii H., Ashida H. Theobromine enhances the conversion of white adipocytes into beige adipocytes in a PPAR γactivation-dependent manner. J. Nutr. Biochem. 2022;100:108898. doi: 10.1016/j.jnutbio.2021.108898. [DOI] [PubMed] [Google Scholar]
- 213.Wei D., Wu S., Liu J., Zhang X., Guan X., Gao L., Xu Z. Theobromine ameliorates nonalcoholic fatty liver disease by regulating hepatic lipid metabolism via mTOR signaling pathway in vivo and in vitro. Can. J. Physiol. Pharmacol. 2021;99:775–785. doi: 10.1139/cjpp-2020-0259. [DOI] [PubMed] [Google Scholar]
- 214.Janitschke D., Lauer A.A., Bachmann C.M., Winkler J., Griebsch L.V., Pilz S.M., Theiss E.L., Grimm H.S., Hartmann T., Grimm M.O.W. Methylxanthines induce a change in the AD/neurodegeneration- linked lipid profile in neuroblastoma cells. Int. J. Mol. Sci. 2022;23:2295. doi: 10.3390/ijms23042295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rebollo-Hernanz M., Aguilera Y., Martin-Cabrejas M.A., Gonzalez de Mejia E. Phytochemicals from the cocoa shell modulate mitochondrial function, lipid and glucose metabolism in hepatocytes via activation of FGF21/ERK, AKT, and mTOR pathways. Antioxidants. 2022;11:136. doi: 10.3390/antiox11010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fernández-Fernández L., Esteban G., Giralt M., Valente T., Bolea I., Solé M., Sun P., Benítez S., Morelló J.R., Reguant J., et al. Catecholaminergic and cholinergic systems of mouse brain are modulated by LMN diet, rich in theobromine polyphenols and polyunsaturated fatty acids. Food Funct. 2015;6:1251–1260. doi: 10.1039/C5FO00052A. [DOI] [PubMed] [Google Scholar]
- 217.Gu R., Shi Y., Huang W., Lao C., Zou Z., Pan S., Huang Z. Theobromine mitigates IL-1β-induced oxidative stress, inflammatory response, and degradation of type II collagen in human chondrocytes. Int. Immunopharmacol. 2020;82:106226. doi: 10.1016/j.intimp.2020.106226. [DOI] [PubMed] [Google Scholar]
- 218.Sarriá B., Gomez-Juaristi M., Martínez López S., García Cordero J., Bravo L., Mateos Briz M.R. Cocoa colonic phenolic metabolites are related to HDL-cholesterol raising effects and methylxanthine metabolites and insoluble dietary fibre to anti-inflammatory and hypoglycemic effects in humans. PeerJ. 2020;8:e9953. doi: 10.7717/peerj.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rodríguez-Rodríguez P., Ragusky K., Phuthong S., Ruvira S., Ramiro-Cortijo D., Cañas S., Rebollo-Hernanz M., Morales M.D., López de Pablo A.L., Martín-Cabrejas M.A., et al. Vasoactive properties of a cocoa shell extract: Mechanism of action and effect on endothelial dysfunction in aged rats. Antioxidants. 2022;11:429. doi: 10.3390/antiox11020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Talbot C.P.J., Mensink R.P., Smolders L., Bakeroot V., Plat J. Theobromine does not affect fasting and postprandial HDL cholesterol efflux capacity, while it decreases fasting miR-92a levels in humans. Mol. Nutr. Food Res. 2018;62:e1800027. doi: 10.1002/mnfr.201800027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Smolders L., Mensink R.P., van den Driessche J.J., Joris P.J., Plat J. Theobromine consumption does not improve fasting and postprandial vascular function in overweight and obese subjects. Eur. J. Nutr. 2019;58:981–987. doi: 10.1007/s00394-018-1612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cova I., Leta V., Mariani C., Pantoni L., Pomati S. Exploring cocoa properties: Is theobromine a cognitive modulator? Psychopharmacology. 2019;236:561–572. doi: 10.1007/s00213-019-5172-0. [DOI] [PubMed] [Google Scholar]
- 223.Shi D., Daly J.W. Chronic effects of xanthines on levels of central receptors in mice. Cell. Mol. Neurobiol. 1999;19:719–732. doi: 10.1023/A:1006901005925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.He R., Xie G., Yao X.-S., Kurihara H. Effect of cocoa tea (Camellia ptilophylla) co-administrated with green tea on ambulatory behaviors. Biosci. Biotechnol. Biochem. 2009;73:957–960. doi: 10.1271/bbb.80815. [DOI] [PubMed] [Google Scholar]
- 225.Mitchell E.S., Slettenaar M., vd Meer N., Transler C., Jans L., Quadt F., Berry M. Differential contributions of theobromine and caffeine on mood, psychomotor performance and blood pressure. Physiol. Behav. 2011;104:816–822. doi: 10.1016/j.physbeh.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 226.Travassos M., Santana I., Baldeiras I., Tsolaki M., Gkatzima O., Sermin G., Yener G.G., Simonsen A., Hasselbalch S.G.F., Kapaki E., et al. Does caffeine consumption modify cerebrospinal fluid amyloid-β levels in patients with Alzheimer’s disease? J. Alzheimer Dis. 2015;47:1069–1078. doi: 10.3233/JAD-150374. [DOI] [PubMed] [Google Scholar]
- 227.Mendiola-Precoma J., Padilla K., Rodríguez-Cruz A., Berumen L.C., Miledi R., García-Alcocer G. Theobromine-induced changes in A1 purinergic receptor gene expression and distribution in a rat brain Alzheimer’s disease model. J. Alzheimer Dis. 2017;55:1273–1283. doi: 10.3233/JAD-160569. [DOI] [PubMed] [Google Scholar]
- 228.Martın-Pelaez S., Camps-Bossacoma M., Massot-Cladera M., Rigo-Adrover M., Franch A., Perez-Cano F.J., Castell M. Effect of cocoa’s theobromine on intestinal microbiota of rats. Mol. Nutr. Food Res. 2017;61:1700238. doi: 10.1002/mnfr.201700238. [DOI] [PubMed] [Google Scholar]
- 229.Rolta R., Salaria D., Sharma B., Awofisayo O., Fadare O.A., Sharma S., Patel C.N., Kumar V., Sourirajan A., Baumler D.J., et al. Methylxanthines as potential inhibitor of SARS-CoV-2: An In Silico Approach. Curr. Pharmacol. Rep. 2022;8:149–170. doi: 10.1007/s40495-021-00276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the article.


