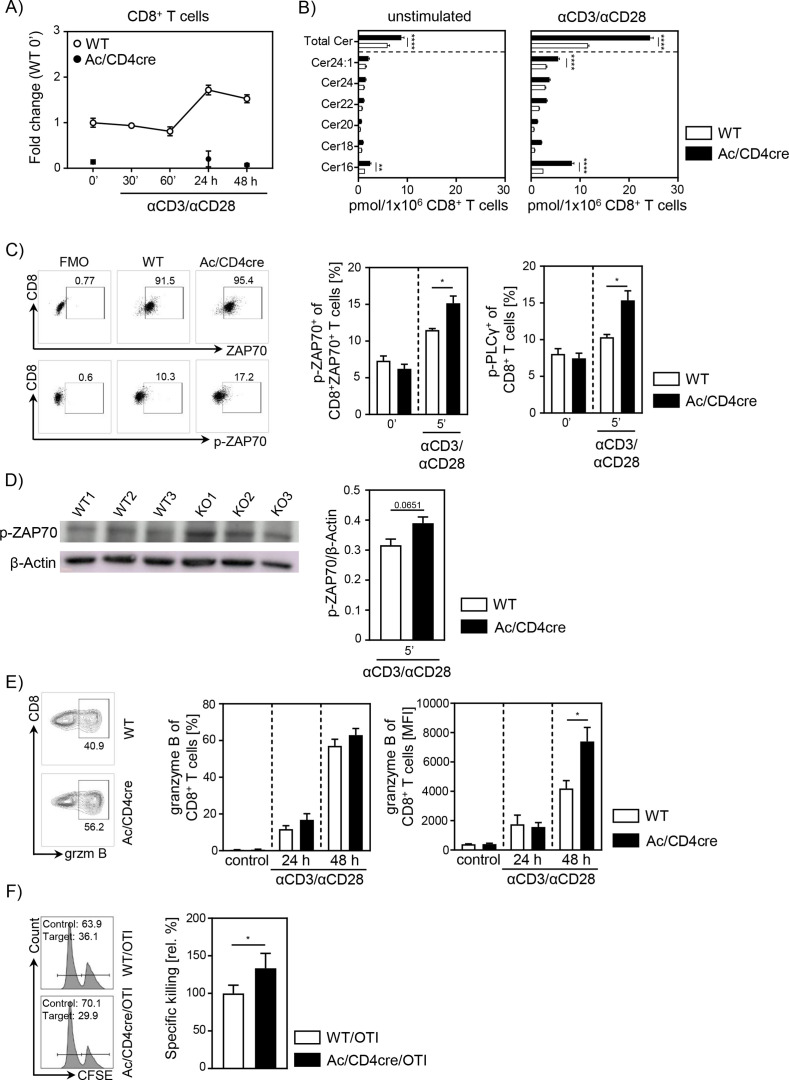
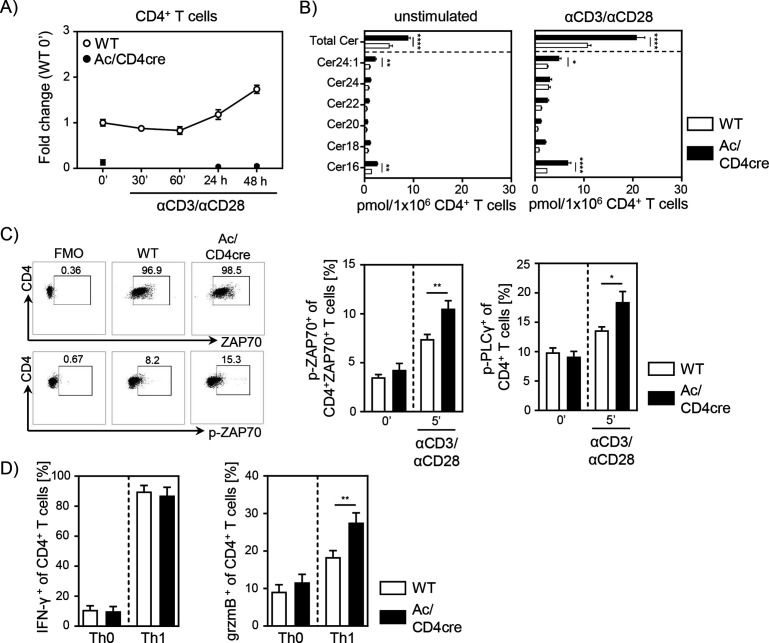
Figure 6. Acid ceramidase (Ac)-deficient CD8+ T cells have elevated ceramide levels and show increased activation in vitro.
(A) Isolated CD8+ T cells from Asah1fl/fl/Cd4cre/+ mice (Ac/CD4cre) and Asah1fl/fl/Cd4+/+ littermates (wildtype [WT]) where either left unstimulated or stimulated with anti-CD3 and anti-CD28 for indicated time points. mRNA expression of Ac (Asah1) following activation was analyzed by RT-qPCR (n=3–4). (B) Ceramide levels of CD8+ T cells were determined by mass spectrometry (n=4). (C) For T cell receptor signaling analysis, splenocytes from Ac/CD4cre and WT mice were left unstimulated (0’) or stimulated with anti-CD3 and anti-CD28 for 5 (5’) min. Afterward, samples were analyzed for phospho-ZAP70 of gated ZAP70+CD8+ T cells and phospho-PLCγ of gated CD8+ T cells by flow cytometry (n=4). Representative dot plots and fluorescence minus one (FMOs) for phospho-ZAP70 are shown in the left panel. (D) Western blot analysis of phospho-ZAP70 expression of CD8+ T cells from Ac/CD4cre and WT mice after 5 min of stimulation with anti-CD3 and anti-CD28 (n=3). (E) CD8+ T cells were left untreated as control or stimulated for 24 or 48 hr and analyzed for granzyme B expression by flow cytometry (n=5–8). Representative contour plots are shown in the left panel. (F) Specific killing of antigen-specific cytotoxic lymphocytes from Ac/CD4cre/OTI mice and WT controls was assessed (n=8–9). Representative histograms are shown in the left panel. Data are depicted as mean ± SEM. Statistical analysis was performed by two-way ANOVA with Sidak’s multiple comparisons, Mann-Whitney U-test, or Student’s t-test. (*p<0.05, **p<0.01, ****p<0.0001).