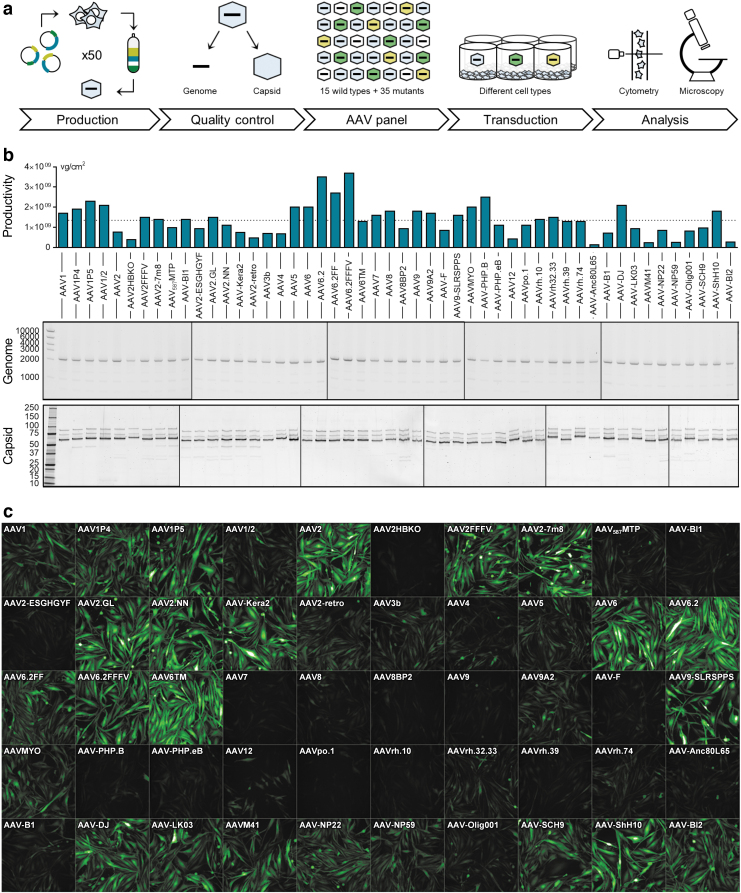
Figure 1.
AAV panel: Production, quality control, and screening procedure. (a) Workflow of the production and usage of the AAV panel. Fifty AAV capsid variants harboring a CMV-eGFP expression cassette were individually produced and purified over iodixanol gradients. After confirming the quality of all viral batches, vectors were arrayed on 96-well plates and applied for the transduction of various cell types. Following a 1- to 3-day incubation, the percentage of GFP-expressing cells was quantified by high-content confocal imaging and, in some instances, flow cytometry. (b) The production efficiency of each AAV variant was determined by ddPCR and is shown as vg per cm2 of culture area. The dotted line indicates the average production efficiency across all batches. AAV genome integrity (expected size: 2,141 bp) was assessed by agarose gel electrophoresis using 1 × 1010 copies of the extracted scAAV genome per lane. Axis labeling: base pairs (middle panel). Capsid protein identity and purity were assessed by SDS-PAGE and Oriole staining. Axis labeling: kDa (lower panel). (c) AAV panel layout and exemplary high-content imaging output for MIO-M1 cells, 3 days after transduction. AAV, adeno-associated virus; ddPCR, droplet digital polymerase chain reaction; sc, self-complementary; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.