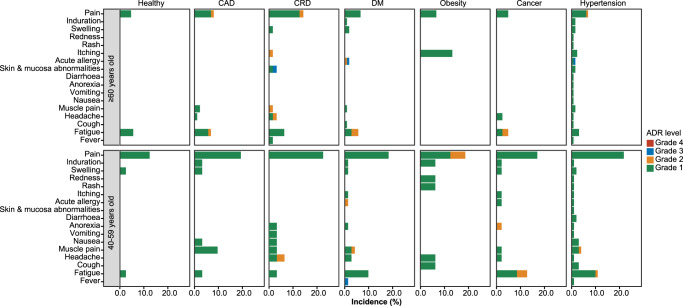
Fig. 2. Incidence of adverse events reported within 14 days post the first dose and the second dose of the vaccination in the safety population, across age groups.
n = 969 study participants. Adverse events post 14 days of first dose and the second dose of the vaccination were collected and graded according to the China National Medical Products Administration guidelines. The histogram shown the incidence of adverse events happened in the 40–60 and ≥60 years old groups. Green, grade 1; orange, grade 2; blue, grade 3; Red, grade 4.