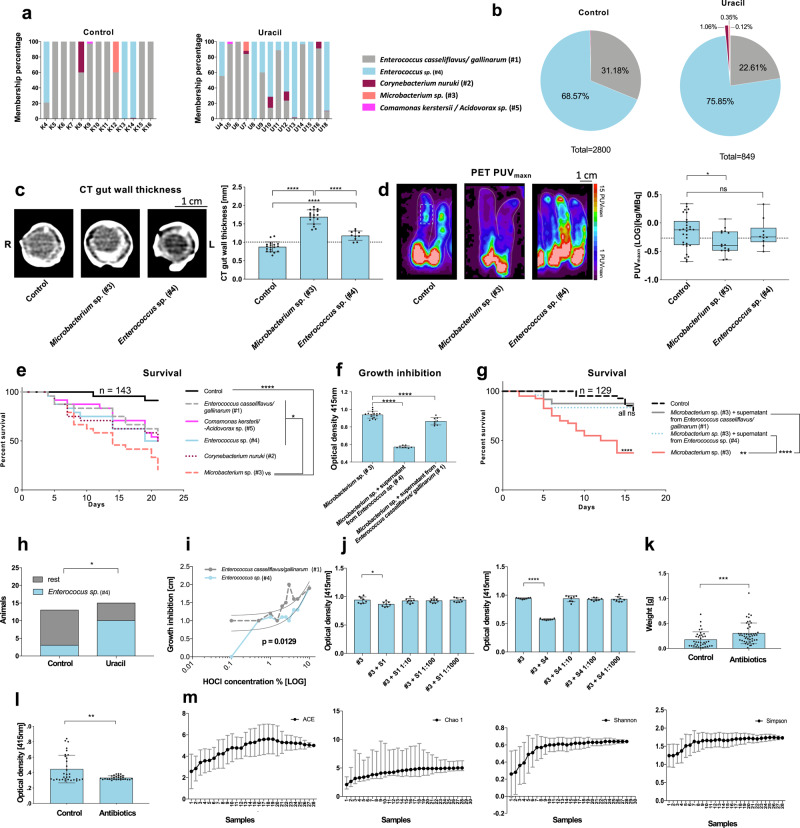
Fig. 9. Discrimination between intestinal bacterial pathogens and mutualists in Manduca sexta by multimodal imaging.
a, b The bacterial composition of fecal bacteria from control or uracil-fed animals (after 20.5 h). c, d Animals fed with the isolated pathogenic Microbacterium sp. (#3) differed significantly from control animals in terms of CT gut wall thickness (n = 45, one-way ANOVA, F(2,42) = 117.5, R2 = 0.8484, P < 0.0001) and PET PUVmaxn (two-tailed t test, P = 0.0437, no adjustments), whereas symbiotic Enterococcus sp. (#4) differed only slightly in terms of CT gut wall thickness and not in PET PUVmaxn (two-tailed t test, P = 0.8334, no adjustments). Dashed lines indicate threshold values. e Microbacterium sp. is considered a pathogen because it reduced the survival of M. sexta significantly more than the other isolated bacteria. f Both Enterococcus spp. (containing a suppressive component against the Microbacterium sp.) suppressed the growth of the Microbacterium sp (n = 32, one-way ANOVA, F(2,29) = 285.4, R2 = 0.9516, P < 0.0001). g Supernatant of the Enterococcus spp. rescued animals fed with Microbacterium sp. Therefore, the enterococci were defined as immunoprotective symbionts. h Although there was a total reduction in the CFU count after uracil treatment, significantly more Enterococcus sp. (#4) positive samples were found in animals treated with uracil, two-sided Chi-square-test, P = 0.0211. The reason for that finding could be the relative insensitivity of Enterococcus sp. #4 against HOCl in comparison to Enterococcus casseliflavus/gallinarum (#1), i dose–response testing revealed an elevation difference: F(1,17) = 7.711. P = 0.0129. j Supernatant from both Enterococcus spp. inhibited the growth of Microbacterium sp. (#3), indicated through less measurable optical density, #3 + S1: n = 40, one-way ANOVA, F(4,35) = 4.043, R2 = 0.316, P = 0.0085 and #3 + S4: n = 40, one-way ANOVA, F(4,35) = 185.9, R2 = 0.9551, P < 0.0001. k Animals treated with penicillin grow significantly faster, indicating the pleiotropic effect of bacterial maintenance for M. sexta, two-tailed Mann Whitney test, P = 0.0004, no adjustments. l The clearance of gut bacteria through penicillin treatment was confirmed using fecal pellets’ optical density measurements, two-tailed t-test, P = 0.0013 no adjustments. m Rarefaction curves showed an adequate sampling of the low-diversity bacterial community in fecal pellets of M. sexta. Cultivable bacteria isolated from the feces of M. sexta (see Tables S11 and S12). Significance levels: ns = P > 0.05, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001. Survival kinetics show the sum of the conducted experiments. Bar charts represent mean and SD. Every data point represents a single animal. Box plots represent 25th-75th percentiles; whiskers represent min–max (show all points), and the centers represent median values. Source data are provided as a Source Data file.