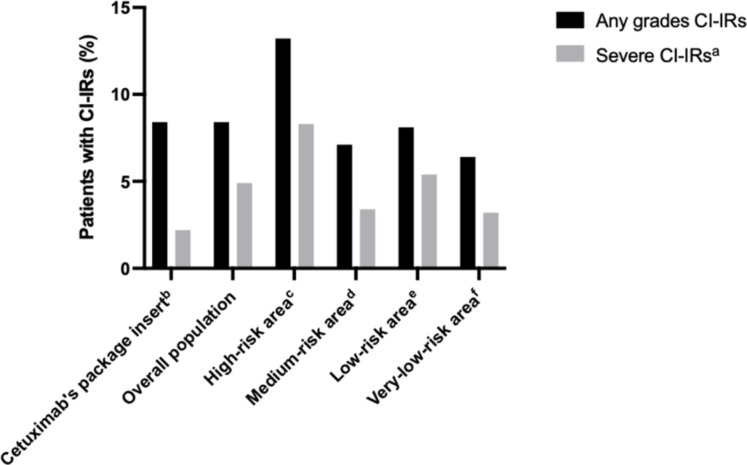
Fig. 2.
Distribution of any grades and severe grades of cetuximab-induced hypersensitivity infusion reactions (CI-IRs) in each area with different Lyme disease risks (N = 117)
CI-IRs, cetuximab-induced hypersensitivity infusion reactions. a defined as grade 3 or grade 4 infusion reactions. b from ImClone Systems Incorporated. U.S. Food and Drug Administration. Erbitux (cetuximab) prescribing information. 2019 [1]. c, d, e, f defined according to estimated mean annual regional incidence rates of Lyme disease from Septfons A, et al. Eurosurveillance. 2019;24(11). doi:10.2807/1560-7917.ES.2019.24.11.1800134 [41] : c > 150 / 100,000 inhabitants; d 50–100 / 100,000 inhabitants; e 20–50 / 100,000 inhabitants; f 5–20 / 100,000 inhabitants