Abstract
The largest US cancer health disparity exists in prostate cancer, with Black men having more than a two-fold increased risk of dying from prostate cancer compared to all other races. This disparity is a result of a complex network of factors including socioeconomic status (SES), environmental exposures, and genetics/biology. Inequity in the US healthcare system has emerged as a major driver of disparity in prostate cancer outcomes and has raised concerns that the actual incidence rates may be higher than current estimates. However, emerging studies argue that equalizing healthcare access will not fully eliminate racial health disparities and highlight the important role of biology. Significant differences have been observed in prostate cancer biology between ancestral groups that may contribute to prostate cancer health disparities. Notably, relative to White men, Black men with prostate cancer exhibit increased androgen receptor signaling, genomic instability, metabolic dysregulation, and inflammatory and cytokine signaling. Immediate actions are needed to increase multi-center, interdisciplinary research to bridge the gap between social and biological determinants of prostate cancer health disparities.
Keywords: African American, Outcomes, Androgen, DNA Damage, Inflammation, Tumor microenvironment, Social determinants
1. Introduction
In 1854, British surgeon-pathologist Sir Henry Thompson described the first case of prostate carcinoma. Today, over 160 years later, prostate cancer has become the most frequently diagnosed cancer among males in 112 countries around the globe. According to the 2020 GLOBOCAN report, a total of 375,304 men worldwide died from prostate cancer-related deaths, with some of the highest mortality rates being recorded in the Caribbean (27.9 deaths per 100,000) and sub-Saharan African (24.8 deaths per 100,000) regions [1]. Prostate cancer has the largest disparities by race of any cancer. In the United States, between 2012 and 2018, the age-adjusted overall incidence rate of prostate cancer was 171.6 per 100,000 Black men compared to 97.7 per 100,000 non-Hispanic White men (1.76-fold higher in Black men), and 53.8 per 100,000 in Asian/Pacific Islanders (3.19-fold higher in Black men). The age-adjusted overall mortality due to prostate cancer in the US between 2015 and 2019 in Black men was the highest in the world; 38.3 per 100, 000 Black men compared to 17.9 per 100,000 in non-Hispanic White men (2.14-fold higher) and 8.8 per 100,000 in Asian/Pacific Islanders (4.35-fold higher) (Fig. 1) [2,3]. Importantly, age-specific comparisons using Surveillance, Epidemiology, and End Results Program (SEER) data illustrate significant racial differences in prostate cancer incidence during different periods of life (See Table 1 for age-stratified incidence and mortality rates). These notable differences across races in both incidence and mortality rates lead us to hypothesize an underlying role for biological drivers in addition to socioeconomic and environmental factors.
Fig. 1.
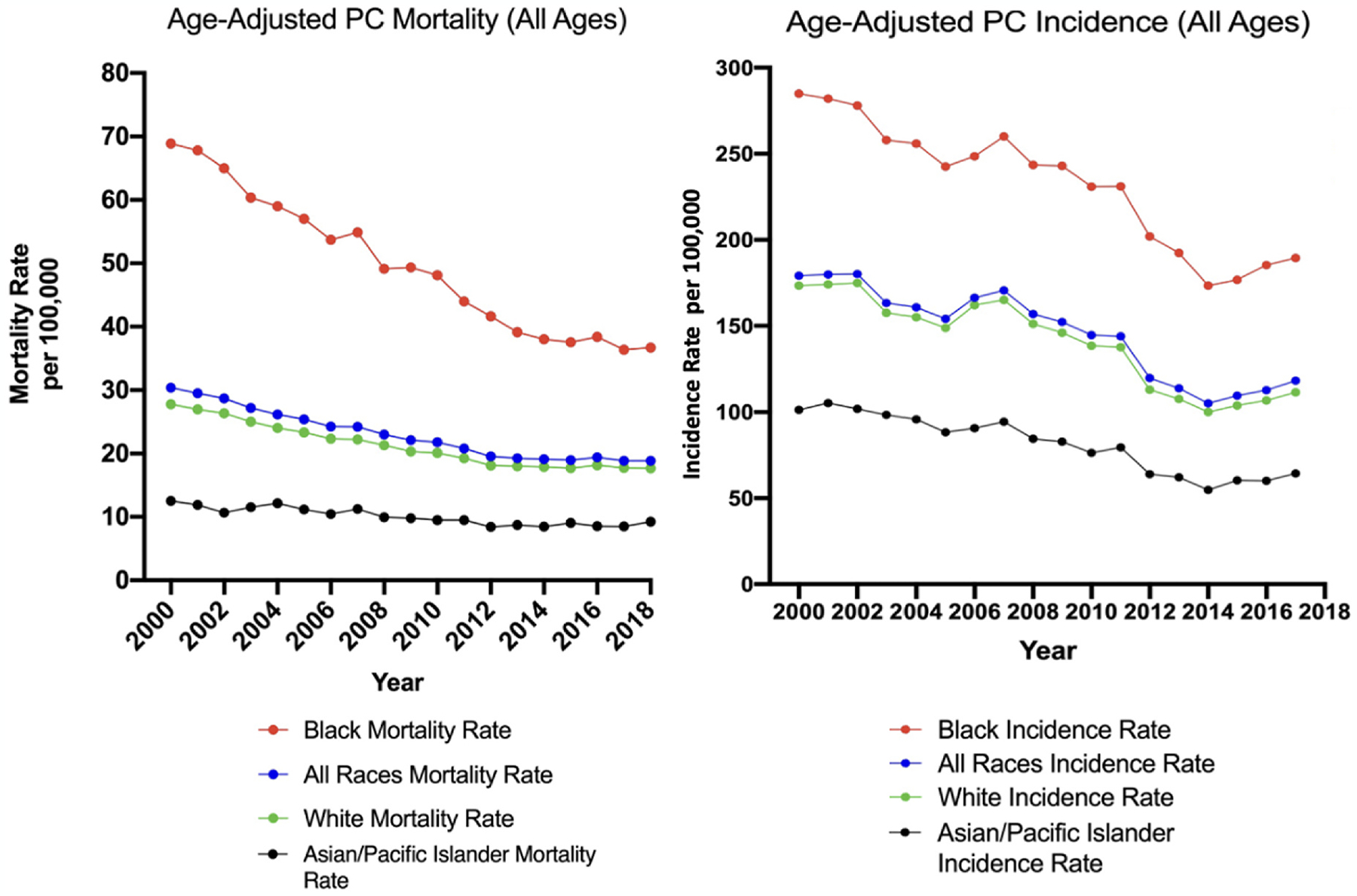
Annual SEER prostate cancer (PC) mortality rates (left) and incidence rates (right) of selected racial groups (Black, White, Asian/Pacific Islander, All races) in the US per 100,000 people [3]. Prostate cancer incidence and mortality rates have declined over the past twenty years for each racial group, though rates of prostate cancer incidence and mortality remain significantly higher for Black Americans relative to all other racial groups.
Table 1.
Left panel: age-stratified average SEER prostate cancer incidence and mortality rates (per 100,000 people) of selected racial groups (Non-Hispanic White, Black (includes Hispanic), Hispanic (any race), and Asian/Pacific Islander (includes Hispanic)) in the US from 2012–2018 [3]. Right panel: the ratios of prostate cancer incidence and mortality rates in each racial group, relative to Non-Hispanic White incidence/mortality rates. Notably, the Black:White disparities in prostate cancer incidence (2.01 to 1.62 to 1.39) and mortality (2.78 to 2.58 to 1.96) decrease in severity as the groups increase in age, suggesting the racial disparity is more severe among younger (< 65 years old) Black men. SEER prostate cancer incidence and mortality rates are lower in Hispanic men compared to White men (all ratios ≤ 1), and lowest in Asian/Pacific Islander men compared to White men (all ratios ≤ 0.70).
| 2012–2018 Average PC Incidence* | Ratios of PC Incidence, Relative to Non-Hispanic White | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| White(Non-Hispanic) | Black (includes Hispanic) | Hispanic(any race) | Asian/Pacific Islander (includes Hispanic) | White: White Ratio | Black: White Ratio | Hispanic: White Ratio | Asian/PI: White Ratio | ||
| *Rate per 100,000 | Ages 40–64 | 123.9 | 248.9 | 95.4 | 53.3 | 1.0 | 2.01 | 0.77 | 0.43 |
| Ages 65–74 | 651.6 | 1055.5 | 581.6 | 376.7 | 1.0 | 1.62 | 0.89 | 0.58 | |
| Ages 75+ | 492.5 | 686.7 | 482.6 | 337.2 | 1.0 | 1.39 | 0.98 | 0.68 | |
| 2012–2018 Average PC Mortality* | Ratios of PC Mortality, Relative to Non-Hispanic White | ||||||||
| White(Non-Hispanic) | Black (includes Hispanic) | Hispanic(any race) | Asian/Pacific Islander (includes Hispanic) | White: White Ratio | Black: White Ratio | Hispanic: White Ratio | Asian/PI: White Ratio | ||
| *Rate per 100,000 | Ages 40–64 | 4.1 | 11.4 | 3.7 | 1.6 | 1.0 | 2.78 | 0.90 | 0.40 |
| Ages 65–74 | 48.2 | 124.5 | 43.2 | 20.2 | 1.0 | 2.58 | 0.90 | 0.42 | |
| Ages 75+ | 224.5 | 440.5 | 195.9 | 113.8 | 1.0 | 1.96 | 0.87 | 0.51 | |
In this mini review, we will first briefly examine the non-biological determinants of prostate cancer health disparities to facilitate a discussion on the interplay between the socioeconomic inequities and genomics in driving prostate cancer disparities. We aim to provide an up-to-date summary of the biological drivers of prostate cancer by race/ethnicity and discuss how future studies can improve precision oncology and alleviate prostate cancer health disparities.
2. Socioeconomic and healthcare inequities are key determinants of prostate cancer disparities, but do not fully explain observed disparities
A multitude of data supports the hypothesis that systemic discrimination and socioeconomic differences are major drivers of prostate cancer disparities.
2.1. Incidence and mortality rates by race and socioeconomic status
In the United States, Black men are 1.76 times more likely to be diagnosed and 2.14 times more likely to die from prostate cancer compared to White men. Black men are also more likely to have a more advanced stage of prostate cancer at the time of diagnosis [4,5]. According to United States health coverage statistics, in 2019, 9.6% of Black men were uninsured, compared to 16.7% of Hispanic men and 5.2% of non-Hispanic White men [6]. Notably, while both Black men and Hispanic men experience significant barriers in accessing healthcare, prostate cancer incidence and mortality levels in Black men are the highest of all races/ethnic group. he same disparity is not observed in Hispanic men (Table 1). This is perhaps partially due to the socioeconomic barriers that are not identical for Black and Hispanic men. The socioeconomic effects/lifestyle consequences may also vary by race and therefore may affect Black men more than Hispanic men. Importantly, prostate cancer incidence and mortality in Hispanic men is not only lower compared to Black men but is also lower compared to non-Hispanic White men, hinting at a role for biological factors in driving disparate outcomes. If access to care and socioeconomic status solely drove prostate cancer disparities, then we would not expect lower prostate cancer mortality in Hispanic men compared to Black and White men (Table 1).
Two analyses of prostate cancer outcomes showed prostate cancer mortality rates decrease for White men as SES increases, but mortality rates do not decrease for Black men as SES increases [7,8]. This provides further evidence, though geographically limited, that SES alone does not fully account for disparate outcomes in prostate cancer and highlights the importance of other sociological and biological factors (Fig. 2). We conclude there is a biological component contributing to disparate outcomes in prostate cancer and review key racial-biological differences in sections 4–7 of this review.
Fig. 2.
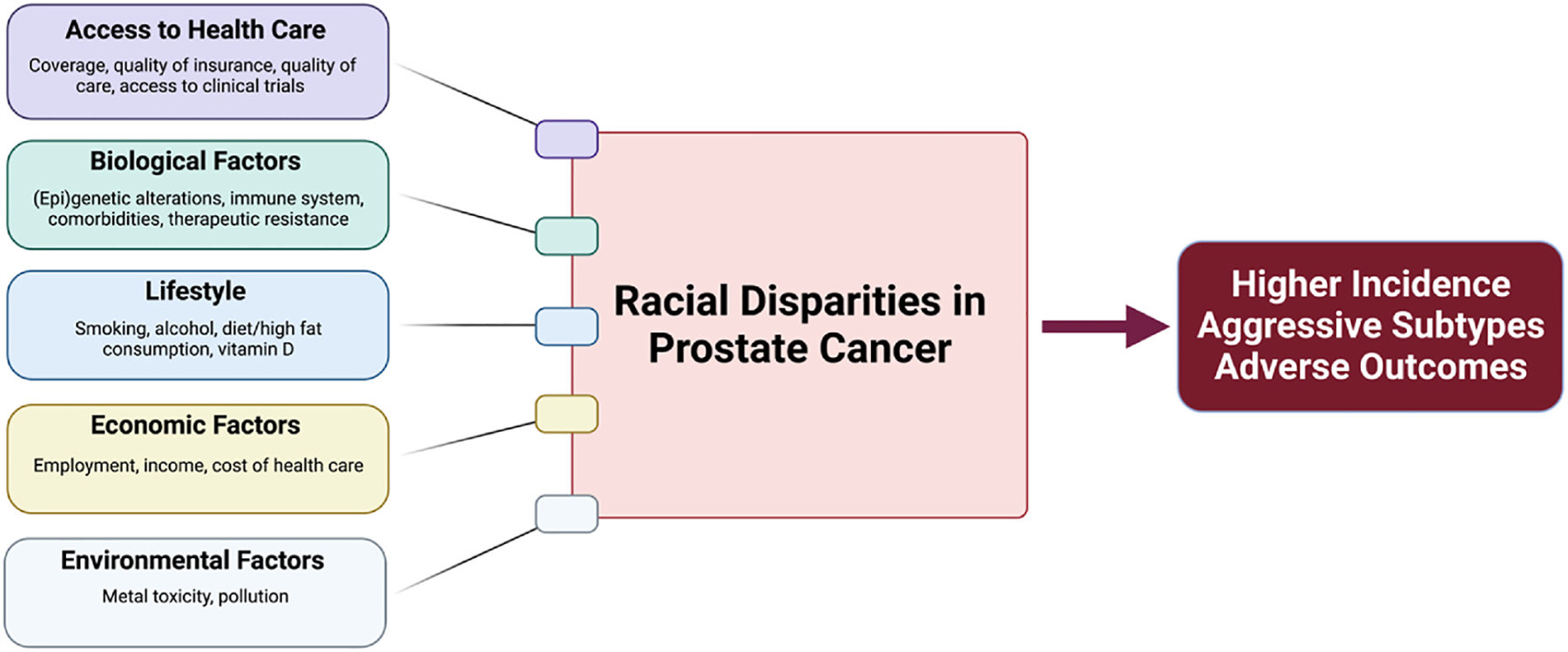
Determinants of Racial Disparities in Prostate Cancer. A multitude of complex parameters including social factors (e.g., access to healthcare, diet), economic factors (e.g., cost of healthcare), environmental factors (e.g., metal toxicity), and biological factors (e.g., family history, genetic and epigenetic alterations) collectively contribute to increased incidence and mortality rates of prostate cancer in Black men compared to men of other races/ethnicities.
2.2. Survival rates in an equal-access medical system
To determine whether an equal-access medical system can attenuate the disparities observed by race, multiple retrospective studies have focused on the Veterans Affairs (VA) United States healthcare system. Riviere and team recently carried out one of the largest analyses to date using a centralized database of >20 million veterans. They assembled a cohort of 60,035 veterans diagnosed with prostate cancer (30.3% Black men and 69.7% non-Hispanic White men) and found that the overall survival rate in Black men was similar to that of non-Hispanic White men [9]. Similar observations have been made in other smaller VA cohorts by other investigators [10–12]. Retrospective studies evaluating the impact of access to healthcare on survival are limited outside of the VA system. A handful of studies have noted that the racial disparity in prostate cancer mortality diminishes among patients age 65+ and among patients with Medicare coverage; however, cohort sizes were small [13, 14]. A multiple-cohort study by Dess et al. with over 300,000 Prostate Cancer patients found that, relative to White men, Black men with nonmetastatic prostate cancer had similar prostate cancer specific mortality in a setting with equal access to care and a standardized treatment regimen [15]. Collectively, these studies highlight that helping to ensure equal access to healthcare for every patient will be a major way to increase survival benefits for all races.
It is important to note that many of these VA-centric studies focused on survival rate and stage at diagnosis in men with prostate cancer and do not provide an estimate of incidence rate. Survival rate is determined by the natural history of the disease (i.e., stage at diagnosis and therapeutic efficacy). Consequently, survival rate is highly sensitive to availability of cancer screening and treatment options. Incidence and mortality rates, on the other hand, are estimates of a disease burden at the population level (i.e., overall veteran population), which is excluded from VA-centric studies.
2.3. Limitations to prostate cancer screening
Incidence rates are also driven by availability of screening. For example, in 2012, the US Preventive Services Task Force (USPSTF) released a recommendation against PSA-based screening for prostate cancer (Grade D recommendation). Following the USPSTF recommendation, the Behavioral Risk Factor Surveillance System reported significant decreases in PSA screening (9.3% among non-Hispanic Whites, 11.6% among non-Hispanic Black, and 7.9% in Hispanics) between 2012 and 2018 [11,12] and concurrently, the incidence ratio of localized prostate cancer in Black men compared to White men increased during this period. A marked reduction was also noted in the number of prostate biopsies performed and in the diagnosis of low-risk, intermediate-risk, and high-risk prostate cancer in the US [13]. Most importantly, a considerably higher prostate cancer mortality rate was observed in Black men in this timeframe [14,16]. Prior studies have illustrated that Black men with prostate cancer were younger at diagnosis and presented with higher PSA levels at diagnosis compared to White men [5]. It is important to note here that prostate cancer health disparities studies often adjust for stage or grade of disease in an attempt to adjust for disparities in screening.
Keeping these limitations in mind, according to the Automated Central Tumor Registry (ACTUR) of the Department of Defense, the incidence of prostate cancer in military men (between 20 and 59 years of age), irrespective of race, continued to be twice that of the general population [17]. Military men with exposure to Agent Orange and other battlefield chemicals often present with more aggressive prostate cancer [18]. Other studies have also reported that Black men are still more likely to have higher Gleason scores and PSA levels than their White counterparts in equal access settings [12,19,20]. These recurrent observations highlight that, even with equal access to care, there is an underlying difference in prostate cancer biology across different races and an early onset of prostate cancer in Black men that warrants further exploration.
3. Interplay between non-biological and biological factors of health disparities and current limitations
Studies have illustrated that disease-related loci in prostate cancer in Black men display higher prevalence of DNA hypermethylation compared to other races [21,22]. However, assessment of how epigenetic imprinting is influenced by socioeconomic status is yet to be determined. As we move forward, it is important to keep in mind that the biological and the non-biological determinants of prostate cancer are two sides of the same coin.
3.1. Limitations in availability of diverse biological specimens
The impact of non-biological determinants of prostate cancer on incidence, mortality, and survival have been the focus of epidemiological research for decades. Substantial advances in geographical information systems (GISs) now provide greater spatial/geographical context to these studies. On the other hand, comprehensive, large-scale studies of biological determinants are far fewer and incomplete, and do not incorporate individual-level socioeconomic status. Importantly, investigation of biological determinants depends on access to biospecimens from a diverse population of patients. The vast majority of previously published studies aimed at identifying biological drivers of prostate cancer health disparities had limited biospecimens from diverse patients. Most frequently, biospecimens are obtained in a clinical setting and require willingness of the patients to participate in clinical trials and/or donating biological specimens to biorepositories for molecular analysis. A major barrier in prostate cancer research is low enrollment of Black men in clinical trials. A recent study by Rencsok and team of 72 global Phase II and IV clinical trials (with 893,378 participants) conducted between 1987 and 2016 found that 96% of total pooled participants were White, while Africans and Caribbeans comprised only 3% of the participants [23]. A similar study looking at metastatic castration resistant prostate cancer (mCRPC) clinical trials within the US also found that Black men were grossly underrepresented in clinical trials, making up a mere 3.3% of the total trial participants (other racial minorities were also vastly underrepresented ≤0.5%) [24]. This lack of participation can be attributed to disproportionate hurdles faced by Black men including, but not limited to: poverty, access to transportation, healthcare, childcare, and knowledge about clinical trials [25]. As others have highlighted, low accrual into clinical trials is a product of lack of access, financial burden of follow-up meetings and/or lab testing not covered by the trial, historical mistrust of the healthcare system, and catchment area of trials amongst other factors [24,26]. Despite advancement in technological tools for rapidly analyzing biospecimens and reduction in sequencing costs, we continue to be limited in our studies due to low access to biospecimens from diverse groups.
This limitation is primely exemplified in a recent seminal genome-wide association study (largest of its kind) carried out by Haiman and colleagues. The researchers calculated a genetic risk score (GRS) of 269 risk variants in 107,247 prostate cancer cases and 127,006 controls and found that the mean GRS in Black men was 2.18 times higher than that of White men while Asian men had 0.73 times lower association than White men [27]. The researchers note that this level of high mean GRS score in Black men has not been consistently observed in other cancers across multi-center studies and is likely seen in prostate cancer because of a strong genetic component to the disease. The group identified 86 novel, independent genetic risk loci of which 32 were significantly associated with prostate cancer in White men. However, only 5 new risk loci were identified for Asian men and only 1 for Black men. Identification of risk loci in Black men was limited due to the cohort size (Black man constituted less than 10% of the cohort) yet again emphasizing the need for enrollment of non-White men in clinical trials to identify additional risk variants.
3.2. Limitations in ancestral characterization
A major confounding factor in understanding the biological drivers of prostate cancer health disparities is the classification of study participants using only self-reported ‘race’ (which is more of a social construct than biology) and not genetic variation or ancestry [28]. An overwhelming majority of the currently available studies lack sequencing-driven genetic ancestry data. A recent genomic study, aimed at understanding how prostate cancer genomes differed by self-reported race and genetic ancestry, found that clinical factors and cancer risk factors differed noticeably by self-reported race and ancestry [29]. Thus, equalizing access to care is unlikely to fully eliminate racial disparities and a more meaningful understanding of the common drivers of prostate cancer in various ancestry groups are critical to eliminating inequities in prostate cancer. Through genetic ancestry analysis, in a recent study, Kittles and colleagues illustrated that some commercially available “African American” prostate cancer cell lines (based on self-identified race), such as E006AA-hT, carry 91% European genetic ancestry [28]. Another widely advertised ‘mixed’ race prostate cancer cell line, 22Rv1, was shown to carry 99% European ancestry. Therefore, as we recognize the need for additional samples and model systems to conduct molecular biology research of health disparities, it is imperative that such models are accurately categorized based on genetic ancestry to facilitate meaningful research.
With these limitations in mind, we now shift our attention to discussing key findings from multiple large-scale comparative studies highlighting differences in gene and pathway alteration across racial groups and their implications.
4. Genetic and molecular basis of health disparities in prostate cancer
Histological and large-scale genomic data illustrate that prostate cancer is highly heterogeneous at the individual and population levels, and this diversity contributes to both phenotypic and functional plasticity. We hypothesize that biological factors play a critical role in the early onset of prostate cancer in Black men and as well as the aggressive nature of the disease (i.e., resistance to available therapeutics). As such, biological factors are critical influencers of prostate cancer incidence, survival, and mortality rate.
4.1. Androgen receptor signaling
The androgen receptor (AR) is a ligand-regulated transcription factor that plays a central role in the development and function of the normal prostate as well as in initiation and progression of prostate cancer (Fig. 3) [30]. Ligands that stimulate AR signaling include testosterone and dihydrotestosterone (DHT).
Fig. 3.
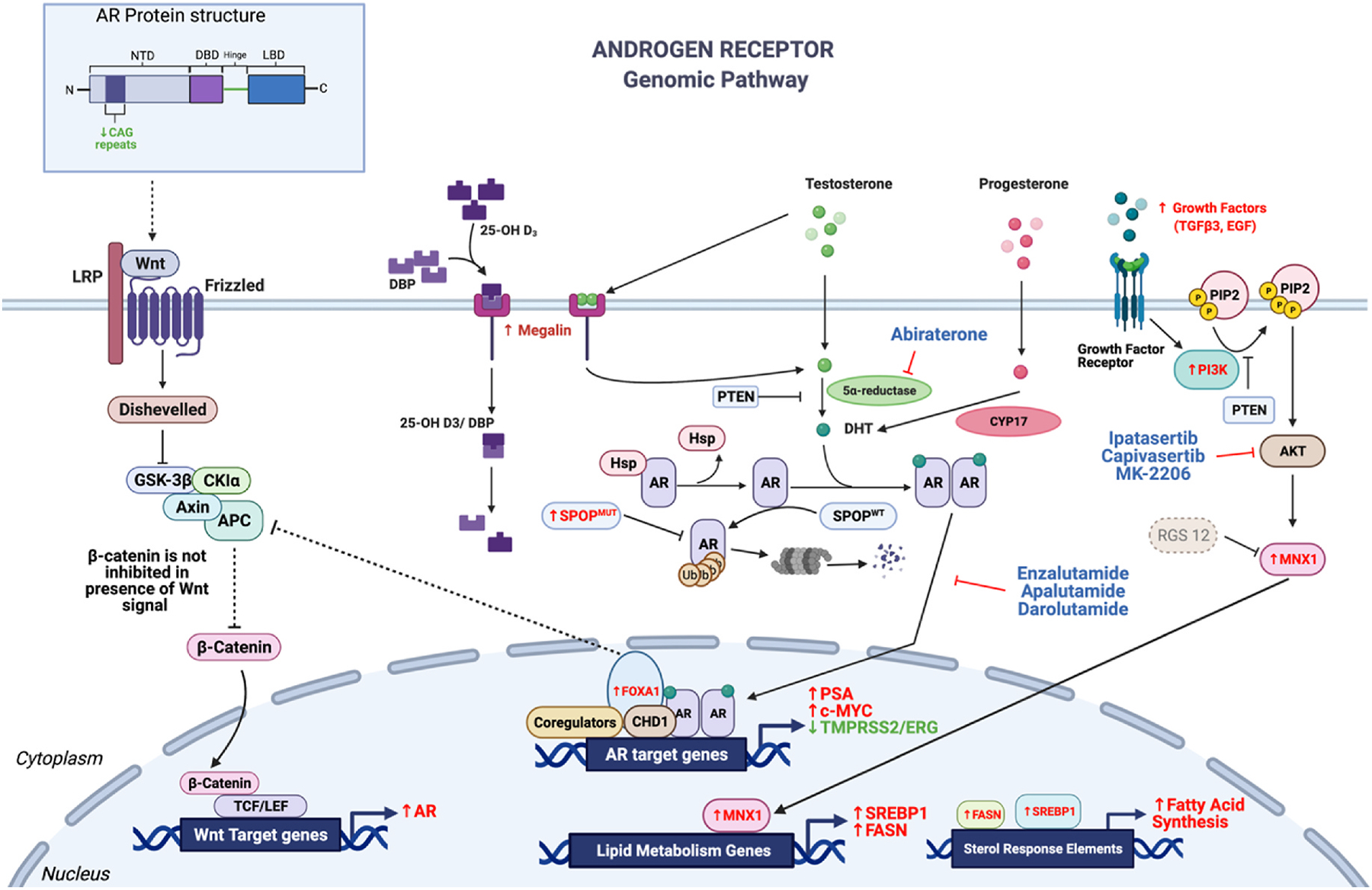
Androgen receptor (AR)-mediated regulation of prostate cancer. Directionality of the arrows (up = increase, down = decrease) and font color (red = increase, green = decrease) indicate the expression level of these drivers in Black men compared to White men with prostate cancer. Canonical AR signaling is mediated through androgen binding AR in the cytoplasm, which leads to a conformational change in AR causing it to dimerize. Upon dimerization, AR translocates to the nucleus and binds to androgen response elements (ARE) to allow transcription of many cancer-related genes. In Black men with prostate cancer, PSA and c-Myc levels are higher and TMPRSS2/ERG fusion rates are lower compared to White men with prostate cancer. A non-canonical AR signaling pathway mediated through PI3K and Akt also regulates AR signaling via phosphorylation. Many components of the PI3K pathway are upregulated in Black men with prostate cancer, including increased serum levels of growth factors, increased MNX1 activity, and preferential deletion of RGS12, leading to higher transcription of lipid metabolism genes such as SREBP1 and FASN.
4.1.1. Increased AR protein and AR ligand in Black men
Several studies have reported that Black men have higher (as much as 4.9% higher) free testosterone levels compared to White men [31]. Studies have also reported increased AR protein levels in prostate cancer in Black men relative to White men, even after normalization for Gleason score [32,33] as well as increased somatic and germline hyper-mutation of AR in Black men with prostate cancer [34].
4.1.2. Presence of shorter CAG repeats in Black men
A notable alteration in the AR gene is the length of the polymorphic cytosine, adenine, guanine (CAG) repeat sequence in Exon 1 of AR, which encodes the N-terminal transactivation domain. CAG repeat/PolyQ tract length is inversely correlated with AR transcriptional activity (Fig. 3), risk of developing prostate cancer, and being diagnosed with a more advanced stage of prostate cancer [35,36]. Multiple clinical studies have unequivocally established that the length of CAG repeats in men of African ancestry is significantly shorter compared to other races (with and without prostate cancer) [37–39]. Shorter length of CAG repeats have been correlated with more aggressive disease in some studies [37,40,41].
4.1.3. AR splice variants
Emergence of post-transcriptional AR splice variants (i.e., AR-v7 and AR-v567) following castration have been linked to resistance and aggressive disease in White men [42], while the role of the AR splice variants in prostate cancer in Black men remains elusive. In a recent study, Armstrong and group assessed AR-v7 status in a cohort of 98 mCRPC using one of the two assays [43]. Within this cohort, 23% of Black men and 29% of White men tested positive for the AR-v7 splice variant. In a second study conducted by Tagawa and colleagues, AR splice variant was detectable in 90% of White men and 86% of Black men with mCRPC [44]. Both studies are confounded by low representation of Black men in these studies (5 Black men in the Armstrong study and 8 Black men in the Tagawa study).
4.1.4. Vitamin D deficiency in Black men
Interestingly, studies have highlighted strong association between Vitamin D deficiency and increased prostate cancer aggressiveness, mortality, and disparity [45–48]. The expression of megalin (LRP2), an endocytic membrane receptor that imports globulin-bound Vitamin D, is significantly increased in men of West African ancestry [49] (Fig. 3). Megalin-mediated import of androgens, which drive prostate cancer tumorigenesis via AR, likely highlights a compensatory response in prostate tissue to Vitamin D deficiency in Black men. This critical role for Vitamin D in driving prostate cancer aggressiveness was further highlighted in a recent race-stratified study that illustrated that Vitamin D intake above Recommended Dietary Allowance is inversely associated with high risk and high grade prostate cancer in Black men but not in White men [50].
Collectively, these studies highlight an important role for the AR signaling axis in driving prostate cancer health disparities. Yet, many questions remain unanswered. Does AR transcriptional output vary across different ancestral groups? Prior studies have demonstrated that the AR cistrome can be extensively reprogrammed during prostate cancer tumorigenesis and disease progression in White men, however, how AR cistromes vary across ancestral groups is not known.
4.1.5. Clinical response to AR signaling inhibitors in Black men
In the first ever prospective multicenter study stratified by race (NCT01940276), George and colleagues found that, relative to White men with mCRPC, Black men are more likely to have greater and more durable PSA responses to a combination of abiraterone acetate (Zytiga) and prednisone [51]. Abiraterone acetate is a selective, irreversible inhibitor of CYP17 and can suppress adrenal synthesis of androgen precursors as well as in situ steroidogenesis in the tumor microenvironment. In the Abi race trial, the median time to PSA progression in Black men was 16.6 months compared to 11.5 months in White men. Interestingly, radiographic progression-free survival (rPFS) and median overall survival (OS) were the same in Black and White men. Whether this is due to the presence of alternative, ligand independent AR activation mechanisms in prostate cancer in Black men remains to be determined.
4.2. Lower occurrence of TMPRSS2-ETS fusion and PTEN deletion in Black men
The most common genomic alteration in prostate cancer is the fusion of the 5′-UTR of TMPRSS2 (21q22) with the 3′-end of ETS family members, such as ERG (21q22), ETV1 (7p21), ETV4 (17q21), or ETV5 (3q27). Multiple studies have confirmed that the TMPRSS2:ERG gene fusion is significantly less common in prostate cancer in Black men (29.3% Black vs. 39.6% White) [52]. Notably, TMPRSS2:ERG fusion positive prostate cancer in White men is often enriched for PTEN loss. Similar to TMPRSS2:ERG fusion, the loss of PTEN is far less common in Black men (11.5% in Black men vs. 30.2% in White men) [52]. A recent study in Black men from South Africa, reported that low frequency of TMPRSS2:ERG fusion was significantly associated with early onset of low-grade prostate cancer presentation, with higher expression from distal ERG junction coordinates [53]. Further studies are warranted to understand why (and how) the frequency of these fusion events are reduced in Black men and how that contributes to early onset of prostate cancer.
4.3. Increased alteration in FOXA1 in Black men
Forkhead box A1 (FOXA1) is a pioneer transcription factor that promotes AR binding and transcriptional activity and is required for normal development of the prostate gland [54,55]. In a cohort of 2393 primary prostate cancer patients, (2109 White, 204 Black, 80 Asian), FOXA1 mutations were shown to be more frequent in Black men compared to White men (18.6% vs. 11.9%) [56]. The functional consequences of these mutations remain to be fully determined, although they likely result in an increased activation of the AR signaling axis. Notably, a recent study of Asian men reported high frequency of FOXA1 missense mutation that resulted in non-functional protein or indel that resulted in frame-shift deletion [57]. Whether these genomic changes are associated with improved prostate cancer survival in Asian men has not been determined.
4.4. Increased alterations in SPOP and CHD1 in Black men
Somatic missense mutations in the speckle-type pox virus and zinc finger (POZ) protein (SPOP) gene occur frequently and early in prostate carcinogenesis and define a genomically distinct class of prostate cancer. SPOP is an adaptor for the Cullin 3 (Cul3)/Rbx1 E3 ubiquitin ligase system and is important for ubiquitination and subsequent degradation of several oncoproteins, including AR and many of its co-regulators [58–60]. Prostate cancer-associated SPOP mutations disrupt its ability to bind substrates and promote their degradation, resulting in a dominant-negative protein that causes dysregulation of several major signaling pathways and cellular processes in prostate cancer [60]. SPOP mutations frequently co-occur with CHD1 (chromodomain helicase DNA binding protein 1) deletions but are mutually exclusive with TMPRSS2-ERG rearrangements [59]. Loss of CHD1 results in altered chromatin occupancy of AR and promotes oncogenic AR-driven transcription [61]. Emerging data suggest that CHD1 deletion events are higher in Black men than in White men [62]. Multiple groups have reported higher frequency of SPOP mutations in prostate cancer in Black men compared to White men, [52,63,64], though other studies have observed similar frequency of SPOP mutations in Black men and White men [65].
The higher frequency of SPOP mutations and potentially of CHD1 deletions in prostate cancer in Black men have multiple implications. As SPOP mutations occur early in prostate tumorigenesis, they may contribute to early onset of an aggressive prostate cancer observed clinically in Black men. Second, in clinical settings, SPOP mutation and CHD1 deletion may be used as biomarkers. Both SPOP and CHD1 are important modulators of DNA damage repair and alterations in these genes can result in increased DNA damage repair via the error-prone non-homologous end joining (NHEJ) pathway, which increases mutational burden in prostate cancer [66,67]. Recently, poly-ADP ribose polymerase (PARP) inhibitors (olaparib, rucaparib, and niraparib) were approved for the treatment of homologous repair-deficient mCRPC. However, PARP inhibitor treatment decisions are currently solely driven by mutations in a small panel of homologous recombination repair (HRR) genes. Prior preclinical studies have suggested that SPOP mutation and/or CHD1 loss can result in increased sensitivity to PARP and other DNA damage response inhibitors (such as (ataxia telangiectasia and Rad3-related) ATR inhibitors) [68]. Retrospective clinical data analysis evaluating performance of PARP inhibitors across racial groups have not been published yet. Further studies are warranted to determine whether SPOP mutation and CHD1 loss can be used as additional biomarkers in decision making steps for PARP inhibitors and other DNA damage repair (DDR) inhibitors.
5. Racial disparities in DNA damage and genomic instability in prostate cancer
Germline mutations in DNA repair genes are associated with higher risk of developing prostate cancer and more aggressive prostate cancer [69–71]. In primary and metastatic prostate cancer, germline mutations are common in BRCA1 and BRCA2 genes; loss-of-function mutations in BRCA1/2 lead to a deficiency in error-free homologous recombination (HR) repair (Fig. 4). Importantly, BRCA mutations are currently the best predictor of response to platinum-based chemotherapy [72] and PARP inhibitors [73]. Multiple recent studies reported a higher rate of BRCA2 (2.8-fold higher) mutations in Black men compared to White men, including one study where protein-truncating unique BRCA2 mutations were identified in Black men with early-onset of prostate cancer [74,75]. Aside from mutations in HRR genes, frequent mutations have also been reported in nucleotide excision repair pathway (NER) genes in Black men with prostate cancer (at least one mutation in NER genes in 89% of tumors) [76]. On the other hand, mutations in mismatch repair pathway (MMR) genes, MutS homolog 2 and 6 (MSH2 and MSH6) [77] and TP53 [29] were less common in Black men. Aside from DNA repair pathway gene mutations, dysfunctional telomeres are a major source for genomic instability. The guanine rich nucleotides in telomeres are susceptible to oxidative damage by reactive oxygen species [78] and mutations in DNA damage repair pathways can result in telomere shortening [79]. Further, leukocyte telomere length was reported to be shorter in high grade prostate cancer in Black men and was associated with aggressive disease and biochemical recurrence after radical prostatectomy and radio-therapy [80,81].
Fig. 4.
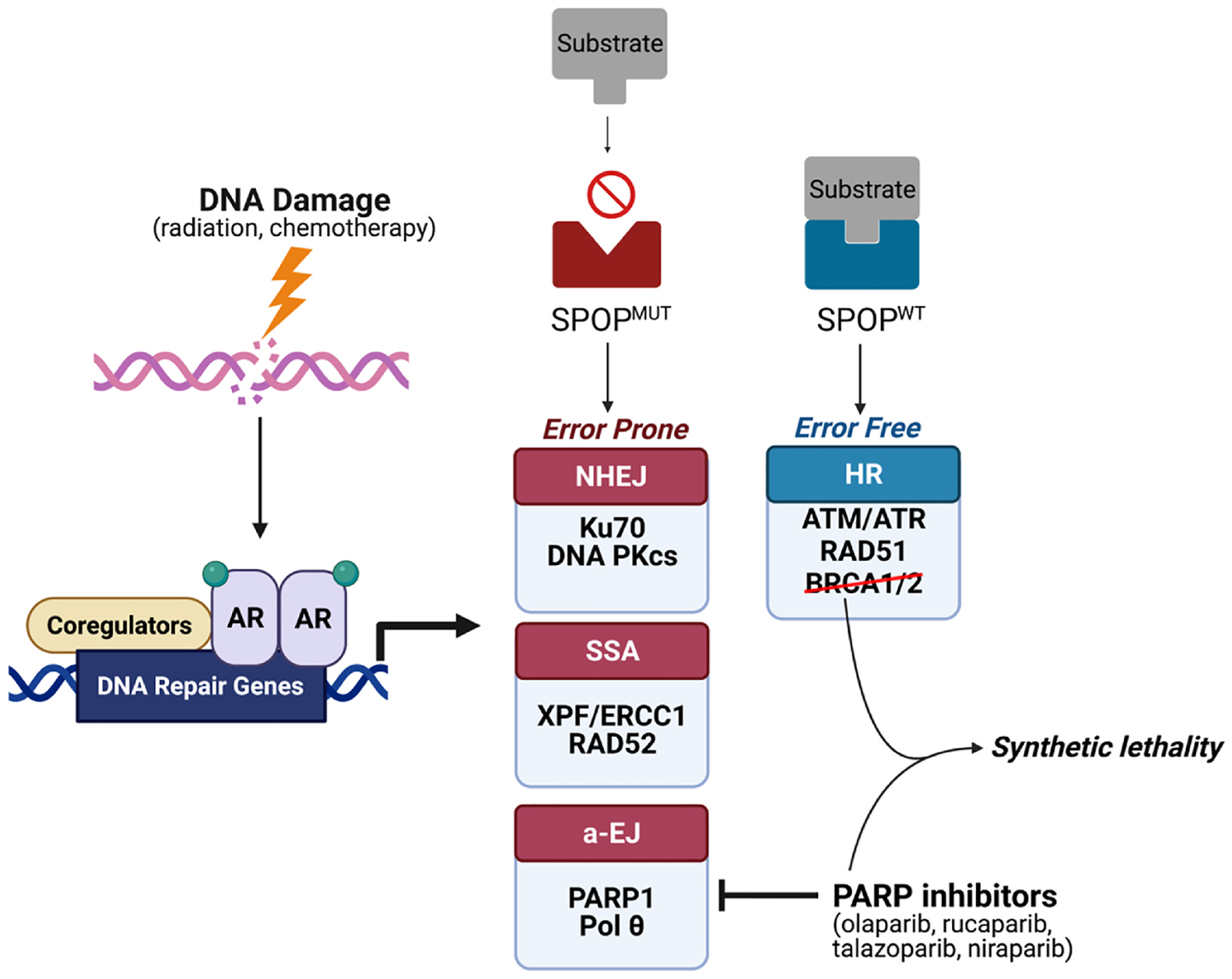
Mutations in DNA repair genes occur more frequently in Black men with prostate cancer compared to White men (22.5% vs 15.6%). DNA damage in prostate cells causes activation of DNA repair pathways such as homologous recombination (HR), non-homologous end joining (NHEJ), alternative end joining (a-EJ), and single-strand annealing (SSA) via AR signaling. NHEJ, a-EJ, and SSA are considered error-prone pathways, whereas HR is considered error-free. Black men have increased rates of SPOP mutations (SPOPmut) compared to White men. SPOP mutations increase the rate of NHEJ, leading to more error-prone DNA repair. PARP inhibitors have been approved for use in patients with deleterious HR gene (BRCA1/2) mutations. Given the similarity between HR gene mutations and SPOP mutations (both resulting in error-prone DNA damage repair), PARP inhibitors have the potential to also benefit prostate cancer patients with SPOP mutations.
Genetic alterations can also result from a combination of exogenous insults, such as X-rays, ultraviolet light, and various chemicals (i.e., polycyclic aromatic hydrocarbons (PAHs)), and endogenous assaults from reactive oxygen species (ROS) and other reactive metabolites. Recent prospective studies have shown that high levels of PAH-DNA adducts (from cigarette smoke exposure and/or charred meat) significantly increase the risk of prostate cancer development in Black men but not in White men [82,83]. Studies have also reported race specific polymorphisms in several enzymes (CYP1A1 Ile462Val, CYP1B1 A119S and L432V, mEH Tyr113His and His139Arg, CYP3A4 A(−392)G) and glutathione S-transferases (GSTs)) that are critical for metabolism of PAHs and detoxication of the primary and dihydrodiol epoxides of PAHs [84]. Notably, polymorphism in GST (GSTT1 null genotype) increased risk of biochemical recurrence of prostate cancer in Black men [84]. In sum, increased genomic instability in Black men with prostate cancer driven by modulators of DNA damage recognition or repair protein and/or exogenous factors highlight potential utility of biomarkers in delivering targeted therapy which can improve overall survival of Black men with prostate cancer.
6. Racial disparities in metabolic dysregulation in aggressive prostate cancer
High-fat diet and obesity are strongly associated with prostate cancer incidence and progression, while increased lipogenesis and uptake of exogenous lipids are linked to prostate cancer aggressiveness and recurrence [85–88]. Increased prostatic total and free fatty acids correlate with higher occurrence, progression, and worse prostate cancer outcomes in Black men, compared to other racial groups [89]. Rapidly proliferating cancer cells also upregulate de novo lipogenesis, even in the presence of exogenous fatty acid, to provide lipids for membrane formation, protein lipidation, intratumoral androgen synthesis, and to support energy production via β-oxidation [90]. Relative to White men, Black men with prostate cancer have increased expression of essential de novo lipogenesis genes such as SREBP1/2, FASN, stearoyl-CoA desaturases, α-methylacyl-CoA racemase, and acetyl-CoA carboxylase (Fig. 3) [91–93]. Overexpression of SREBP1 and FASN is associated with tumor aggressiveness, poor clinical outcomes, and drug resistance through dysregulation of lipid metabolism [94]. We have previously illustrated that MNX1, a homeobox transcription factor, is expressed at a significantly higher level in Black men with prostate cancer compared to their White counterparts, and MNX1 upregulates lipid synthesis by stimulating expression of SREBP1 and fatty acid synthetase (Fig. 3) [95]. In addition, MNX1 and de novo lipogenesis pathways are regulated by AKT signaling, which is almost universally upregulated in advanced prostate cancer and linked to prostate cancer progression, worse patient outcomes, and therapeutic resistance [96, 97]. This upregulation is frequently a consequence of loss of PTEN, a dual protein/lipid phosphatase and an important negative regulator of PI3K-AKT-mTOR signaling. Notably, in Black men with prostate cancer, PTEN loss is less common (11.5% in Black men vs. 30.2% in White men) [52]. Thus, alternative, novel mechanisms likely result in the constitutive activation of PI3K-AKT-mTOR signaling. Recent mapping of the RNA splicing landscape of prostate cancer across racial populations showed differential splicing events in highly prevalent cancer-associated genes and pathways [98]. Specific splice variants of PIK3CD, FGFR3, TSC2 and RASGRP2 have been shown to enhance AKT/mTOR signaling and increase proliferative and invasive capacity of prostate cancer in Black men [98], suggesting differential splicing is a driver of PI3K-AKT-mTOR signaling. Notably, RGS12, a recently identified negative regulator of MNX1 and AKT signaling that is located on chromosome 4p16.3, is preferentially deleted in prostate cancer in Black men [99]. Thus, altered genomic and epigenomic signaling, resulting in enhanced activation of oncogenic signaling and alternative metabolism can potentially drive tumor cell transformation into a more aggressive prostate cancer. Importantly, multiple preclinical studies of inhibitors targeting lipogenesis have consistently illustrated a decrease in AR protein and its transcriptional output [100,101], suggesting that targeting tumor fat metabolism will likely increase efficacy of current FDA-approved anti-androgens (such as enzalutamide and abiraterone) and taxanes, particularly in Black men with prostate cancer. This latter hypothesis remains to be tested under a clinical trial setting.
7. Racial disparities and the prostate tumor microenvironment
Socioeconomic factors (such as stress and diet) can result in heightened oxidative stress and increased inflammatory reactivity, which contributes disease risk and burden. Recent studies have reported significant differences in immune and inflammatory pathways between prostate cancer in Black and White patients and suggest that increased inflammation in the tumor microenvironment (TME) of prostate cancer in Black men is a driver of disparate clinical outcomes [102]. Pro-inflammatory cytokine genes, including CXCR4, IL6, IL8, TNF, IL1β, and MMP9 (Fig. 5) show higher expression in prostate cancer in Black men compared to White men [103,104]. CXCR4–CXCL12 signaling is interconnected with central prostate cancer oncogenic signaling and the promotion of prostate cancer metastasis, highlighting its potential as a biomarker for inflammation and metastasis in prostate cancer in Black men [105]. Interleukin-6 (IL6) and IL8 are known activators of AR signaling and are associated with resistance to androgen deprivation therapy in prostate cancer [104]. TNF-alpha and IL1β activate both CXCR4 and matrix metalloproteinases (MMPs) including MMP9, leading to epithelial-mesenchymal transition (EMT) and metastasis in prostate cancer cells [106]. Moreover, Black men with prostate cancer have higher serum levels of TGFβ3 compared to White men. Notably, serum TGFβ3 levels were higher in Black men without prostate cancer than levels in White men with prostate cancer. Collectively, these studies highlight significant differences in tumor microenvironment between Black and White men.
Fig. 5.
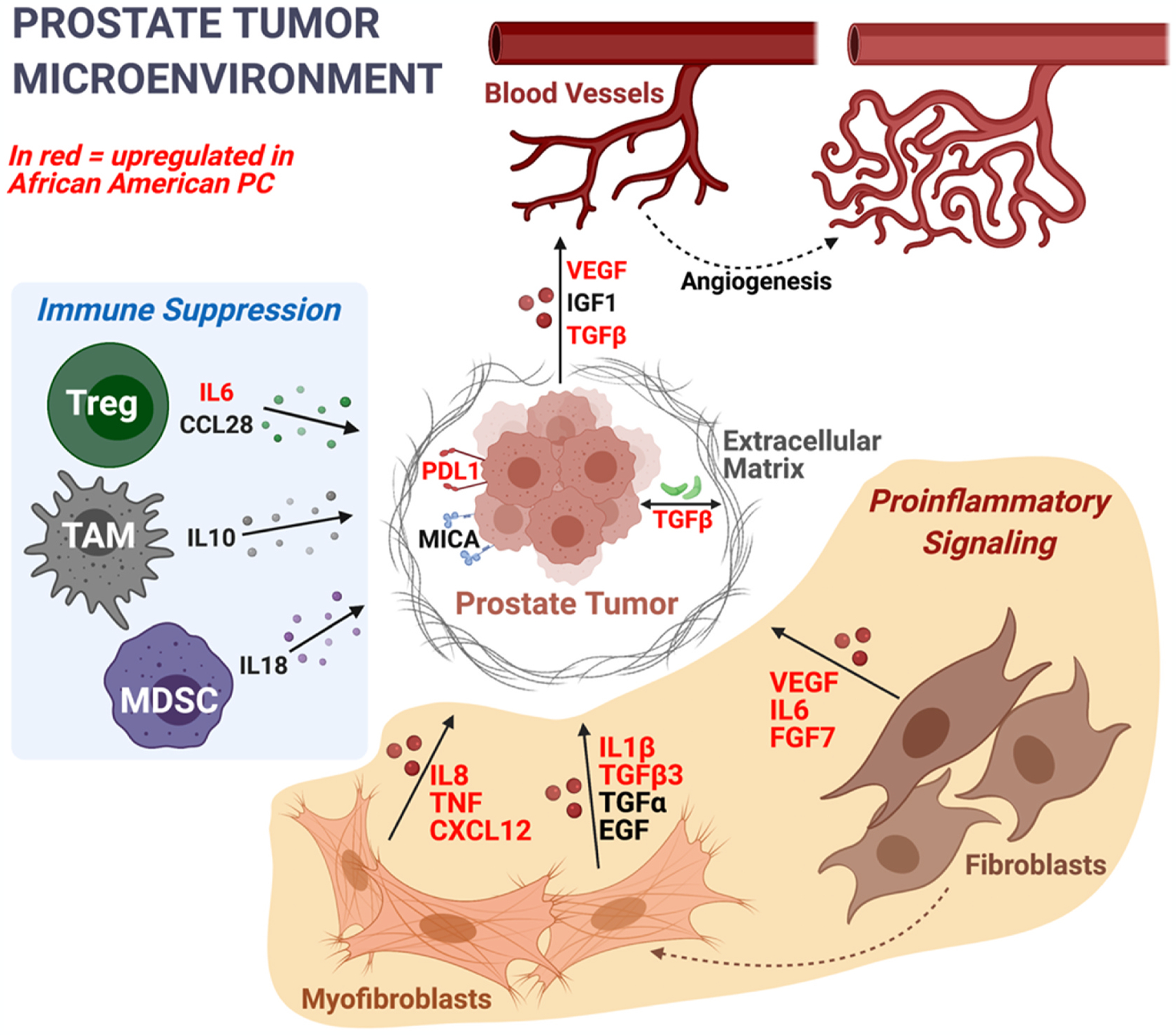
Most upregulated immune/inflammation genes in prostate cancer in Black men have proinflammatory functions, as opposed to immunosuppressive. Genes in red are upregulated in prostate cancer in Black men relative to White men. Some key cell subpopulations involved in immunosuppressive signaling are shown, including regulatory T cells (Treg), tumor-associated macrophages (TAM), and myeloid-derived suppressor cells (MDSC). During carcinogenesis, stromal fibroblasts differentiate into cancer-associated myofibroblasts. As key members of the prostate tumor microenvironment, both fibroblasts and myofibroblasts express proinflammatory cytokines, such as IL6, IL8, TNF, TGFβ, and IL1β (all of which are expressed at higher rates in prostate cancer in Black men compared to prostate cancer in White men).
Consistent with these observed biological differences within the TME, oncogenic signaling, and immunologic pathways, recent retrospective analysis of PROCEED (PROVENGE Registry for the Observation, Collection, and Evaluation of Experience Data; NCT01306890) found increased overall survival in Black men with mCRPC compared with White men with prostate cancer when treated with sipuleucel-T, an autologous cellular immunotherapy [107,108]. Inhibitors of transforming growth factors (TGFs) in mCRPC are being actively evaluated in clinical trials [109]. Whether TGFβ3-targeting therapies will have a greater benefit in Black men with prostate cancer remains to be seen.
The studies highlighted in the above section outline important differences in prostate cancer among men of different ancestry. These differences are likely to affect disease emergence, aggressiveness, detection and response to therapy, and ultimately patient survival.
8. Concluding remarks
Prostate cancer incidence and mortality vary across racial groups, with Black men carrying the greatest burden, the causes of which are complex. In 2015, the Precision Medicine Initiative began, aiming to enhance the understanding of the genetic and environmental determinants of disease in clinical settings across the United States. As discussed in this brief review, ancestry-related differences in genomic alterations, gene expression, epigenetic modifications, tumor metabolism, tumor microenvironment, and immunogenicity are likely contributors of disparities in prostate cancer across racial groups and will undoubtedly impact how we develop new precision therapeutic interventions. Studies on biology and access to healthcare similarly highlight the need for increased representation of men from underserved racial groups, particularly Black men, in translational and clinical research to fully comprehend and appreciate the tumor heterogeneity. This lack of participation in clinical and translational research can be attributed to disparate barriers faced by Black men such as poverty, access to transportation, healthcare, childcare, and clinical trial education [25]. Future studies are warranted using SNP-based ancestry stratification in early-onset and metastatic prostate cancer. Further-more, understanding that prostate cancer in Black men is different from prostate cancer in White men in terms of transcriptome is not enough. To facilitate meaningful clinical advances, we must identify master regulators that drive these differences.
Translational laboratory research often focuses on validating and informing clinical observations using disease models (i.e., providing molecular mechanisms). This research suffers from a severe lack of racially diverse prostate cancer models. Currently, there are approximately 200 prostate cancer cell lines (including parental lines and their derivatives) that originate from White men, and only five from Black men. As noted elsewhere in this review, commercially available models can be incorrectly characterized. Future translational health disparities research will require development of pre-clinical models, such as genetically engineered mouse models, patient derived xenograft (PDX) models, and most recently, ex-vivo organoid models, which will facilitate and improve the development and testing of experimental therapeutics. In addition to generating novel models, researchers must be willing to share newly established models with the community to maximize impact. Despite reports of approximately 120 prostate cancer PDX models in the literature, only a handful are available to the research community to facilitate translational research, and none are from men of African ancestry [110]. Establishing national biorepositories can significantly empower the community of prostate cancer health disparities investigators. To this end, we draw our reader’s attention to the following resource for novel cancer models supported by the Nation Cancer Institute: https://portal.pdxnetwork.org/.
It is abundantly clear that racial disparities in prostate cancer are a result of a complex system. We further propose that biological factors play a critical role in early onset of prostate cancer in Black men. As such, biological factors are critical influencers of prostate cancer incidence, age at onset, survival, and mortality rate. However, such biological differences are not solely genetic or predetermined, but rather can be a result of physiological response to psychosocial stressors, such as racism and segregation. This phenomenon can be further explained by the “Weathering Hypothesis” which states exposure to chronic stress can cause premature decline in one’s physical health [111,112]. Additionally, Black men experience higher allostatic load (the cumulative burden of chronic stress and life events), and a 2006 study by Coker et al., showed higher John Henryism (in which individuals cope with stress with over-performance) scores indicating high-effort coping that may be associated with an increase in prostate cancer risk [112,113]. These interactions, which ultimately affect physical health, can partially explain the racial disparity in PC, though further work is required to quantify causality. Therefore, future prostate cancer health disparities research must prioritize interdisciplinary multicenter studies to promote meaningful and quality health disparities research. An emerging body of work in cancer health disparities research strongly supports an important role for socioeconomic status on epigenetic imprinting, higher inflammatory signature [114], changes in DNA methylation status [115], and epigenetic aging [116]. These and other studies raise a horrifying concern that systemic racism and social inequalities not only kill cancer patients due to lack of access to healthcare but importantly, structural racism may leave behind heritable imprints. Unfortunately, studies bridging social determinants with epigenetic processes remain sparse or completely lacking for certain racial groups. It is broadly recognized that the drivers of health disparities in prostate cancer are multifaceted and complex. To truly tackle this problem, researchers must emerge from their silos to develop adequate infrastructure to conduct multi-center, multi-omics studies that incorporate multidimensional geocoded census data and precise, individual-level data [117]. Ultimately, funding agencies, prostate cancer researchers, healthcare providers, and patients must recognize all aspects of prostate cancer health disparities and collaborate to drive more comprehensive studies under relevant conditions and models to mitigate prostate cancer health disparities.
Significance.
Black men in the US are 1.76 times more likely to be diagnosed with prostate cancer and 2.14 times more likely to die from prostate cancer compared to White men. Ensuring equal access to quality healthcare is vital in addressing racial disparities in prostate cancer but is unlikely to completely eliminate these disparities, as significant differences have been observed in prostate cancer biology between various ancestral groups. Multi-center, interdisciplinary research is urgently needed to bridge the gap between the social determinants and biological factors of health disparities in prostate cancer. Increasing racial diversity in pre-clinical (patient-derived cell and xenograft) models and clinical trials is necessary to further the field’s understanding of the intra-racial and inter-racial heterogeneity of prostate carcinogenesis.
Acknowledgements
Figs. 2–5 created with BioRender.com.
Grant support
D.L. and C.M. are supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number T32GM136554 and T32GM008231. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. N.M., M.I., and S.K. are supported by the Prostate Cancer Foundation, the National Cancer Institute U54-CA233223, and the Department of Defense PCRP IDA W81XWH-18-1-0288 and PCRP HDRA W81XWH2110253.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
D.L., K.R., C.M., A.P., N.M., M.I., report no conflicts of interest. S.K. has received research funding from FGH BioTech.
Abbreviations:
- ATR
ataxia telangiectasia and Rad3-related
- GSTs
glutathione S-transferases
- PAHs
polycyclic aromatic hydrocarbons
- TME
tumor microenvironment
- EMT
epithelial-mesenchymal transition
- TGF
transforming growth factor
- PDX
patient derived xenograft
- AR
androgen receptor
- NEPC
neuroendocrine prostate cancer
- CRPC
castration-resistant prostate cancer
- mCRPC
metastatic castration-resistant prostate cancer
- PARP
Poly(ADP-ribose) polymerase
- NER
nucleotide excision repair
- DDR
DNA damage repair
- MMR
mismatch repair
- HRR
homologous recombination repair
- NHEJ
non-homologous end joining
- UTR
untranslated region
- ETS
erythroblast transformation specific
Footnotes
CRediT authorship contribution statement
Dallin Lowder: Conceptualization, Data curation, Investigation, Writing – original draft, preparation, Visualization. Kinza Rizwan: Conceptualization, Data curation, Investigation, Writing – original draft, preparation, Visualization. Collin McColl: Conceptualization, Data curation, Investigation, Writing – original draft, preparation, Visualization. Alyssa Paparella: Writing – original draft, preparation. Michael Ittmann: Writing - reviewing & editing. Nicholas Mitsiades: Writing - reviewing & editing. Salma Kaochar: Supervision, Conceptualization, Writing - reviewing & editing, Project administration, Funding acquisition.
References
- [1].Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F, Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, CA Cancer J Clin, 2021. [DOI] [PubMed] [Google Scholar]
- [2].A.C. Society, Cancer Facts & Figures for African Americans 2019–2021, American Cancer Society, Atlanta, 2019. [Google Scholar]
- [3].Howlader NNA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, SEER Cancer Statistics Review, 1975–2018, National Cancer Institute, Bethesda, MD, 2020. [Google Scholar]
- [4].Bigler SA, Pound CR, Zhou X, A retrospective study on pathologic features and racial disparities in prostate cancer, Prostate. Cancer 2011 (2011) 239460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tewari A, Horninger W, Pelzer AE, Demers R, Crawford ED, Gamito EJ, Divine G, Johnson CC, Bartsch G, Menon M, Factors contributing to the racial differences in prostate cancer mortality, BJU Int. 96 (2005) 1247–1252. [DOI] [PubMed] [Google Scholar]
- [6].Keisler-Starkey K, Bunch L, Health Insurance Coverage in the United States: 2019, U.S. Census Bureau, Current Population Reports, Washington, DC, 2020, 60–271. [Google Scholar]
- [7].Cheng I, Witte JS, McClure LA, Shema SJ, Cockburn MG, John EM, Clarke CA, Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California, Cancer Causes Control 20 (2009) 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu L, Cozen W, Bernstein L, Ross RK, Deapen D, Changing relationship between socioeconomic status and prostate cancer incidence, J. Natl. Cancer Inst 93 (2001) 705–709. [DOI] [PubMed] [Google Scholar]
- [9].Riviere P, Luterstein E, Kumar A, Vitzthum LK, Deka R, Sarkar RR, Bryant AK, Bruggeman A, Einck JP, Murphy JD, Martínez ME, Rose BS, Survival of African American and non-Hispanic white men with prostate cancer in an equal-access health care system, Cancer 126 (2020) 1683–1690. [DOI] [PubMed] [Google Scholar]
- [10].Daskivich TJ, Kwan L, Dash A, Litwin MS, Racial parity in tumor burden, treatment choice and survival outcomes in men with prostate cancer in the VA healthcare system, Prostate Cancer Prostatic Dis. 18 (2015) 104–109. [DOI] [PubMed] [Google Scholar]
- [11].McKay RR, Sarkar RR, Kumar A, Einck JP, Garraway IP, Lynch JA, Mundt AJ, Murphy JD, Stewart TF, Yamoah K, Rose BS, Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration, Cancer 127 (2021) 403–411. [DOI] [PubMed] [Google Scholar]
- [12].Berger AD, Satagopan J, Lee P, Taneja SS, Osman I, Differences in clinicopathologic features of prostate cancer between black and white patients treated in the 1990s and 2000s, Urology 67 (2006) 120–124. [DOI] [PubMed] [Google Scholar]
- [13].Mahal AR, Mahal BA, Nguyen PL, Yu JB, Prostate cancer outcomes for men aged younger than 65 years with Medicaid versus private insurance, Cancer 124 (2018) 752–759. [DOI] [PubMed] [Google Scholar]
- [14].Liu W, Goodman M, Filson CP, Association of state-level medicaid expansion with treatment of patients with higher-risk prostate cancer, JAMA Netw. Open 3 (2020), e2015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dess RT, Hartman HE, Mahal BA, Soni PD, Jackson WC, Cooperberg MR, Amling CL, Aronson WJ, Kane CJ, Terris MK, Zumsteg ZS, Butler S, Osborne JR, Morgan TM, Mehra R, Salami SS, Kishan AU, Wang C, Schaeffer EM, Roach M 3rd, Pisansky TM, Shipley WU, Freedland SJ, Sandler HM, Halabi S, Feng FY, Dignam JJ, Nguyen PL, Schipper MJ, Spratt DE, Association of black race with prostate cancer-specific and other-cause mortality, JAMA Oncol. 5 (2019) 975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Semega J, Kollar M, Shrider EA, Creamer JF, in: U.S. Census Bureau, C.P. Reports (Ed.), Income and Poverty in the United States: 2019, U.S. Government Publishing Office, Washington, DC, 2020, 60–270. [Google Scholar]
- [17].Zhu K, Devesa SS, Wu H, Zahm SH, Jatoi I, Anderson WF, Peoples GE, Maxwell LG, Granger E, Potter JF, McGlynn KA, Cancer incidence in the U.S. military population: comparison with rates from the SEER program, Cancer Epidemiol. Biomarkers Prev 18 (2009) 1740–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ansbaugh N, Shannon J, Mori M, Farris PE, Garzotto M, Agent Orange as a risk factor for high-grade prostate cancer, Cancer 119 (2013) 2399–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cullen J, Brassell SA, Chen Y, Porter C, L’esperance J, Brand T, McLeod DG, Racial/Ethnic patterns in prostate cancer outcomes in an active surveillance cohort, Prostate. Cancer (2011) 234519, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Powell IJ, Bock CH, Ruterbusch JJ, Sakr W, Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity, J. Urol 183 (2010) 1792–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Martin DN, Starks AM, Ambs S, Biological determinants of health disparities in prostate cancer, Curr. Opin. Oncol 25 (2013) 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Apprey V, Wang S, Tang W, Kittles RA, Southerland WM, Ittmann M, Kwabi-Addo B, Association of genetic ancestry with DNA methylation changes in prostate cancer disparity, Anticancer Res. 39 (2019) 5861–5866. [DOI] [PubMed] [Google Scholar]
- [23].Rencsok EM, Bazzi LA, McKay RR, Huang FW, Friedant A, Vinson J, Peisch S, Zarif JC, Simmons S, Hawthorne K, Villanti P, Kantoff PW, Heath E, George DJ, Mucci LA, Diversity of enrollment in prostate cancer clinical trials: current status and future directions, Cancer Epidemiol. Biomarkers Prev 29 (2020) 1374–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vince R, Spratt DE, Drivers of Racial Disparities in Prostate Cancer Trial Enrollment, Prostate Cancer Prostatic Dis, 2021. [DOI] [PubMed] [Google Scholar]
- [25].Chowdhury-Paulino IM, Ericsson C, Vince R Jr., Spratt DE, George DJ, Mucci LA, Racial Disparities in Prostate Cancer Among Black Men: Epidemiology and Outcomes, Prostate Cancer Prostatic Dis, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McKay RR, Gold T, Zarif JC, Chowdhury-Paulino IM, Friedant A, Gerke T, Grant M, Hawthorne K, Heath E, Huang FW, Jackson MD, Mahal B, Ogbeide O, Paich K, Ragin C, Rencsok EM, Simmons S, Yates C, Vinson J, Kantoff PW, George DJ, Mucci LA, Tackling diversity in prostate cancer clinical trials: a report from the diversity working group of the IRONMAN registry, JCO Global Oncol. 7 (2021) 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Conti DV, Darst BF, Moss LC, Saunders EJ, Sheng X, Chou A, Schumacher FR, Olama AAA, Benlloch S, Dadaev T, Brook MN, Sahimi A, Hoffmann TJ, Takahashi A, Matsuda K, Momozawa Y, Fujita M, Muir K, Lophatananon A, Wan P, Le Marchand L, Wilkens LR, Stevens VL, Gapstur SM, Carter BD, Schleutker J, Tammela TLJ, Sipeky C, Auvinen A, Giles GG, Southey MC, MacInnis RJ, Cybulski C, Wokołorczyk D, Lubiński J, Neal DE, Donovan JL, Hamdy FC, Martin RM, Nordestgaard BG, Nielsen SF, Weischer M, Bojesen SE, Røder MA, Iversen P, Batra J, Chambers S, Moya L, Horvath L, Clements JA, Tilley W, Risbridger GP, Gronberg H, Aly M, Szulkin R, Eklund M, Nordström T, Pashayan N, Dunning AM, Ghoussaini M, Travis RC, Key TJ, Riboli E, Park JY, Sellers TA, Lin HY, Albanes D, Weinstein SJ, Mucci LA, Giovannucci E, Lindstrom S, Kraft P, Hunter DJ, Penney KL, Turman C, Tangen CM, Goodman PJ, Thompson IM, Hamilton RJ, Fleshner NE, Finelli A, Parent M, Stanford JL, Ostrander EA, Geybels MS, Koutros S, Freeman LEB, Stampfer M, Wolk A, Håkansson N, Andriole GL, Hoover RN, Machiela MJ, Sørensen KD, Borre M, Blot WJ, Zheng W, Yeboah ED, Mensah JE, Lu YJ, Zhang HW, Feng N, Mao X, Wu Y, Zhao SC, Sun Z, Thibodeau SN, McDonnell SK, Schaid DJ, West CML, Burnet N, Barnett G, Maier C, Schnoeller T, Luedeke M, Kibel AS, Drake BF, Cussenot O, Cancel-Tassin G, Menegaux F, Truong T, Koudou YA, John EM, Grindedal EM, Maehle L, Khaw KT, Ingles SA, Stern MC, Vega A, Gómez-Caamaño A, Fachal L, Rosenstein BS, Kerns SL, Ostrer H, Teixeira MR, Paulo P, Brandão A, Watya S, Lubwama A, Bensen JT, Fontham ETH, Mohler J, Taylor JA, Kogevinas M, Llorca J, Castaño-Vinyals G, Cannon-Albright L, Teerlink CC, Huff CD, Strom SS, Multigner L, Blanchet P, Brureau L, Kaneva R, Slavov C, Mitev V, Leach RJ, Weaver B, Brenner H, Cuk K, Holleczek B, Saum KU, Klein EA, Hsing AW, Kittles RA, Murphy AB, Logothetis CJ, Kim J, Neuhausen SL, Steele L, Ding YC, Isaacs WB, Nemesure B, Hennis AJM, Carpten J, Pandha H, Michael A, De Ruyck K, De Meerleer G, Ost P, Xu J, Razack A, Lim J, Teo SH, Newcomb LF, Lin DW, Fowke JH, Neslund-Dudas C, Rybicki BA, Gamulin M, Lessel D, Kulis T, Usmani N, Singhal S, Parliament M, Claessens F, Joniau S, Van den Broeck T, Gago-Dominguez M, Castelao JE, Martinez ME, Larkin S, Townsend PA, Aukim-Hastie C, Bush WS, Aldrich MC, Crawford DC, Srivastava S, Cullen JC, Petrovics G, Casey G, Roobol MJ, Jenster G, van Schaik RHN, Hu JJ, Sanderson M, Varma R, McKean-Cowdin R, Torres M, Mancuso N, Berndt SI, Van Den Eeden SK, Easton DF, Chanock SJ, Cook MB, Wiklund F, Nakagawa H, Witte JS, Eeles RA, Kote-Jarai Z, Haiman CA, Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction, Nat. Genet 53 (2021) 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hooker SE, Woods-Burnham L, Bathina M, Lloyd S, Gorjala P, Mitra R, Nonn L, Kimbro KS, Kittles RA, Genetic ancestry analysis reveals misclassification of commonly used cancer cell lines, Cancer Epidemiol. Biomarkers Prev 28 (2019) 1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stopsack KH, Nandakumar S, Arora K, Nguyen B, Vasselman SE, Nweji B, McBride SM, Morris MJ, Rathkopf DE, Slovin SF, Danila DC, Autio KA, Scher HI, Mucci LA, Solit DB, Gonen M, Chen Y, Berger MF, Schultz N, Abida W, Kantoff PW, Differences in prostate cancer genomes by self-reported race: contributions of genetic ancestry, modifiable cancer risk factors, and clinical factors, Clin. Cancer Res 28 (2) (2021) 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lonergan PE, Tindall DJ, Androgen receptor signaling in prostate cancer development and progression, J. Carcinog 10 (2011) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Richard A, Rohrmann S, Zhang L, Eichholzer M, Basaria S, Selvin E, Dobs AS, Kanarek N, Menke A, Nelson WG, Platz EA, Racial variation in sex steroid hormone concentration in black and white men: a meta-analysis, Andrology 2 (2014) 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gaston KE, Kim D, Singh S, Ford OH 3rd, Mohler JL, Racial differences in androgen receptor protein expression in men with clinically localized prostate cancer, J. Urol 170 (2003) 990–993. [DOI] [PubMed] [Google Scholar]
- [33].Kim HS, Moreira DM, Jayachandran J, Gerber L, Banez LL, Vollmer RT, Lark AL, Donovan MJ, Powell D, Khan FM, Freedland SJ, Prostate biopsies from black men express higher levels of aggressive disease biomarkers than prostate biopsies from white men, Prostate Cancer Prostatic Dis. 14 (2011) 262–265. [DOI] [PubMed] [Google Scholar]
- [34].Koochekpour S, Buckles E, Shourideh M, Hu S, Chandra D, Zabaleta J, Attwood K, Androgen receptor mutations and polymorphisms in African American prostate cancer, Int. J. Biol. Sci 10 (2014) 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chamberlain NL, Driver ED, Miesfeld RL, The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function, Nucleic Acids Res. 22 (1994) 3181–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stanford JL, Just JJ, Gibbs M, Wicklund KG, Neal CL, Blumenstein BA, Ostrander EA, Polymorphic repeats in the androgen receptor gene: molecular markers of prostate cancer risk, Cancer Res. 57 (1997) 1194–1198. [PubMed] [Google Scholar]
- [37].Bennett CL, Price DK, Kim S, Liu D, Jovanovic BD, Nathan D, Johnson ME, Montgomery JS, Cude K, Brockbank JC, Sartor O, Figg WD, Racial variation in CAG repeat lengths within the androgen receptor gene among prostate cancer patients of lower socioeconomic status, J. Clin. Oncol 20 (2002) 3599–3604. [DOI] [PubMed] [Google Scholar]
- [38].Sartor O, Zheng Q, Eastham JA, Androgen receptor gene CAG repeat length varies in a race-specific fashion in men without prostate cancer, Urology 53 (1999) 378–380. [DOI] [PubMed] [Google Scholar]
- [39].Gilligan T, Manola J, Sartor O, Weinrich SP, Moul JW, Kantoff PW, Absence of a correlation of androgen receptor gene CAG repeat length and prostate cancer risk in an African-American population, Clin. Prostate. Cancer 3 (2004) 98–103. [DOI] [PubMed] [Google Scholar]
- [40].Giovannucci E, Stampfer MJ, Krithivas K, Brown M, Dahl D, Brufsky A, Talcott J, Hennekens CH, Kantoff PW, The CAG repeat within the androgen receptor gene and its relationship to prostate cancer, Proc. Natl. Acad. Sci. U. S. A 94 (1997) 3320–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Powell IJ, Land SJ, Dey J, Heilbrun LK, Hughes MR, Sakr W, Everson RB, The impact of CAG repeats in exon 1 of the androgen receptor on disease progression after prostatectomy, Cancer 103 (2005) 528–537. [DOI] [PubMed] [Google Scholar]
- [42].Sprenger CC, Plymate SR, The link between androgen receptor splice variants and castration-resistant prostate cancer, Horm Cancer 5 (2014) 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Armstrong AJ, Luo J, Nanus DM, Giannakakou P, Szmulewitz RZ, Danila DC, Healy P, Anand M, Berry WR, Zhang T, Harrison MR, Lu C, Chen Y, Galletti G, Schonhoft JD, Scher HI, Wenstrup R, Tagawa ST, Antonarakis ES, George DJ, Halabi S, Prospective multicenter study of circulating tumor cell AR-V7 and taxane versus hormonal treatment outcomes in metastatic castration-resistant prostate cancer, JCO Precis. Oncol 4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tagawa ST, Antonarakis ES, Gjyrezi A, Galletti G, Kim S, Worroll D, Stewart J, Zaher A, Szatrowski TP, Ballman KV, Kita K, Tasaki S, Bai Y, Portella L, Kirby BJ, Saad F, Eisenberger MA, Nanus DM, Giannakakou P, Expression of AR-V7 and ARv, Clin. Cancer Res 25 (2019) 1880–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Richards Z, Batai K, Farhat R, Shah E, Makowski A, Gann PH, Kittles R, Nonn L, Prostatic compensation of the vitamin D axis in African American men, JCI Insight 2 (2017), e91054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Murphy AB, Nyame Y, Martin IK, Catalona WJ, Hollowell CM, Nadler RB, Kozlowski JM, Perry KT, Kajdacsy-Balla A, Kittles R, Vitamin D deficiency predicts prostate biopsy outcomes, Clin. Cancer Res 20 (2014) 2289–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hollis BW, Marshall DT, Savage SJ, Garrett-Mayer E, Kindy MS, Gattoni-Celli S, Vitamin D3 supplementation, low-risk prostate cancer, and health disparities, J. Steroid Biochem. Mol. Biol 136 (2013) 233–237. [DOI] [PubMed] [Google Scholar]
- [48].Bruce Ames N, William Grant B, Walter Willett C, Does the High Prevalence of Vitamin D Deficiency in African Americans Contribute to Health Disparities? Nutrients 13 (2021) 499, 10.3390/nu13020499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Holt SK, Karyadi DM, Kwon EM, Stanford JL, Nelson PS, Ostrander EA, Association of megalin genetic polymorphisms with prostate cancer risk and prognosis, Clin. Cancer Res 14 (2008) 3823–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Batai Ken, Murphy Adam B, Maria Ruden, Newsome Jennifer, Shah Ebony, Dixon Michael A, Jacobs Elizabeth T, Hollowell Courtney M P,Ahaghotu Chiledum, Kittles Rick A, Race and BMI modify associations of calcium and vitamin D intake with prostate cancer, BMC Cancer 17 (2017) 64, 10.1186/s12885-017-3060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].George DJ, Halabi S, Heath EI, Sartor AO, Sonpavde GP, Das D, Bitting RL, Berry W, Healy P, Anand M, Winters C, Riggan C, Kephart J, Wilder R, Shobe K, Rasmussen J, Milowsky MI, Fleming MT, Bearden J, Goodman M, Zhang T, Harrison MR, McNamara M, Zhang D, LaCroix BL, Kittles RA, Patierno BM, Sibley AB, Patierno SR, Owzar K, Hyslop T, Freedman JA, Armstrong AJ, A Prospective Trial of Abiraterone Acetate Plus Prednisone in Black and White Men with Metastatic Castrate-Resistant Prostate Cancer, Cancer, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yuan J, Kensler KH, Hu Z, Zhang Y, Zhang T, Jiang J, Xu M, Pan Y, Long M, Montone KT, Tanyi JL, Fan Y, Zhang R, Hu X, Rebbeck TR, Zhang L, Integrative comparison of the genomic and transcriptomic landscape between prostate cancer patients of predominantly African or European genetic ancestry, PLoS Genet. 16 (2020), e1008641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Blackburn J, Vecchiarelli S, Heyer EE, Patrick SM, Lyons RJ, Jaratlerdsiri W, van Zyl S, Bornman MSR, Mercer TR, Hayes VM, TMPRSS2-ERG fusions linked to prostate cancer racial health disparities: a focus on Africa, Prostate 79 (2019) 1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Parolia A, Cieslik M, Chu SC, Xiao L, Ouchi T, Zhang Y, Wang X, Vats P, Cao X, Pitchiaya S, Su F, Wang R, Feng FY, Wu YM, Lonigro RJ, Robinson DR, Chinnaiyan AM, Distinct structural classes of activating FOXA1 alterations in advanced prostate cancer, Nature 571 (2019) 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gao N, Ishii K, Mirosevich J, Kuwajima S, Oppenheimer SR, Roberts RL, Jiang M, Yu X, Shappell SB, Caprioli RM, Stoffel M, Hayward SW, Matusik RJ, Forkhead box A1 regulates prostate ductal morphogenesis and promotes epithelial cell maturation, Development 132 (2005) 3431–3443. [DOI] [PubMed] [Google Scholar]
- [56].Mahal BA, Alshalalfa M, Kensler KH, Chowdhury-Paulino I, Kantoff P, Mucci LA, Schaeffer EM, Spratt D, Yamoah K, Nguyen PL, Rebbeck TR, Racial differences in genomic profiling of prostate cancer, N. Engl. J. Med 383 (2020) 1083–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Li J, Xu C, Lee HJ, Ren S, Zi X, Zhang Z, Wang H, Yu Y, Yang C, Gao X, Hou J, Wang L, Yang B, Yang Q, Ye H, Zhou T, Lu X, Wang Y, Qu M, Yang Q, Zhang W, Shah NM, Pehrsson EC, Wang S, Wang Z, Jiang J, Zhu Y, Chen R, Chen H, Zhu F, Lian B, Li X, Zhang Y, Wang C, Wang Y, Xiao G, Jiang J, Yang Y, Liang C, Hou J, Han C, Chen M, Jiang N, Zhang D, Wu S, Yang J, Wang T, Chen Y, Cai J, Yang W, Xu J, Wang S, Gao X, Wang T, Sun Y, A genomic and epigenomic atlas of prostate cancer in Asian populations, Nature 580 (2020) 93–99. [DOI] [PubMed] [Google Scholar]
- [58].Geng C, Rajapakshe K, Shah SS, Shou J, Eedunuri VK, Foley C, Fiskus W, Rajendran M, Chew SA, Zimmermann M, Bond R, He B, Coarfa C, Mitsiades N, Androgen receptor is the key transcriptional mediator of the tumor suppressor SPOP in prostate cancer, Cancer Res. 74 (2014) 5631–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cancer N, Genome atlas research, the molecular taxonomy of primary prostate cancer, Cell 163 (2015) 1011–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Geng C, He B, Xu L, Barbieri CE, Eedunuri VK, Chew SA, Zimmermann M, Bond R, Shou J, Li C, Blattner M, Lonard DM, Demichelis F, Coarfa C, Rubin MA, Zhou P, O’Malley BW, Mitsiades N, Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 6997–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Augello MA, Liu D, Deonarine LD, Robinson BD, Huang D, Stelloo S, Blattner M, Doane AS, Wong EWP, Chen Y, Rubin MA, Beltran H, Elemento O, Bergman AM, Zwart W, Sboner A, Dephoure N, Barbieri CE, CHD1 loss alters AR binding at lineage-specific enhancers and modulates distinct transcriptional programs to drive prostate tumorigenesis, Cancer Cell 35 (2019) 817–819. [DOI] [PubMed] [Google Scholar]
- [62].Diossy M, Tisza V, Li H, Zhou J, Sztupinszki Z, Young D, Nousome D, Kuo C, Chen Y, Ebner R, Sesterhenn IA, Moncur JT, Chesnut GT, Petrovics G, Klus GT, Spisak S, Valcz G, Nuzzo PV, Ribli D, Schina A, Börcsök J, Prosz A, Krzystanek M, Ried T, Szuts D, Kaochar S, Pathania S, D’Andrea AD, Csabai I, Srivastava S, Dobi A, Freedman ML, Szallasi Z, Increased Frequency of CHD1 Deletions in Prostate Cancers of African American Men Is Associated with Distinct Homologous Recombination Deficiency Associated DNA Aberration Profiles, medRxiv, 2021, p. 2021, 2002.2008.21251199. [Google Scholar]
- [63].Khashab T, Le AD, Cohen S, Kaochar S, Dowst H, Noor AB, Zarrin-Khameh N, Brooks MA, Godoy G, Berezina MA, Schwarzbach AE, Scheurer ME, Mims MP, Mitsiades N, Genomic landscape of advanced prostate cancer in racial minority populations: real-world experience in a safety-net hospital oncology clinic, J. Clin. Oncol 39 (2021), 14–14. [Google Scholar]
- [64].Koga Y, Song H, Chalmers ZR, Newberg J, Kim E, Carrot-Zhang J, Piou D, Polak P, Abdulkadir SA, Ziv E, Meyerson M, Frampton GM, Campbell JD, Huang FW, Genomic profiling of prostate cancers from men with African and European ancestry, Clin. Cancer Res 26 (2020) 4651–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Blattner M, Lee DJ, O’Reilly C, Park K, MacDonald TY, Khani F, Turner KR, Chiu YL, Wild PJ, Dolgalev I, Heguy A, Sboner A, Ramazangolu S, Hieronymus H, Sawyers C, Tewari AK, Moch H, Yoon GS, Known YC, Andren O, Fall K, Demichelis F, Mosquera JM, Robinson BD, Barbieri CE, Rubin MA, SPOP mutations in prostate cancer across demographically diverse patient cohorts, Neoplasia 16 (2014) 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Shenoy TR, Boysen G, Wang MY, Xu QZ, Guo W, Koh FM, Wang C, Zhang LZ, Wang Y, Gil V, Aziz S, Christova R, Rodrigues DN, Crespo M, Rescigno P, Tunariu N, Riisnaes R, Zafeiriou Z, Flohr P, Yuan W, Knight E, Swain A, Ramalho-Santos M, Xu DY, de Bono J, Wu H, CHD1 loss sensitizes prostate cancer to DNA damaging therapy by promoting error-prone double-strand break repair, Ann. Oncol 28 (2017) 1495–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Boysen G, Barbieri CE, Prandi D, Blattner M, Chae SS, Dahija A, Nataraj S, Huang D, Marotz C, Xu L, Huang J, Lecca P, Chhangawala S, Liu D, Zhou P, Sboner A, de Bono JS, Demichelis F, Houvras Y, Rubin MA, SPOP mutation leads to genomic instability in prostate cancer, Elife (2015) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Chen YX, Tan LM, Gong JP, Huang MS, Yin JY, Zhang W, Zhou HH, Liu ZQ, Response prediction biomarkers and drug combinations of PARP inhibitors in prostate cancer, Acta Pharmacol. Sin 42 (2021) 1970–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Nombela P, Lozano R, Aytes A, Mateo J, Olmos D, Castro E, BRCA2 and other DDR genes in prostate cancer, Cancers (Basel) (2019) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N, Boysen G, Porta N, Flohr P, Gillman A, Figueiredo I, Paulding C, Seed G, Jain S, Ralph C, Protheroe A, Hussain S, Jones R, Elliott T, McGovern U, Bianchini D, Goodall J, Zafeiriou Z, Williamson CT, Ferraldeschi R, Riisnaes R, Ebbs B, Fowler G, Roda D, Yuan W, Wu YM, Cao X, Brough R, Pemberton H, A’Hern R, Swain A, Kunju LP, Eeles R, Attard G, Lord CJ, Ashworth A, Rubin MA, Knudsen KE, Feng FY, Chinnaiyan AM, Hall E, de Bono JS, DNA-repair defects and olaparib in metastatic prostate cancer, N. Engl. J. Med 373 (2015) 1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Messina C, Cattrini C, Soldato D, Vallome G, Caffo O, Castro E, Olmos D, Boccardo F, Zanardi E, BRCA mutations in prostate cancer: prognostic and predictive implications, JAMA Oncol. 2020 (2020) 4986365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Pomerantz MM, Spisak S, Jia L, Cronin AM, Csabai I, Ledet E, Sartor AO, Rainville I, O’Connor EP, Herbert ZT, Szallasi Z, Oh WK, Kantoff PW, Garber JE, Schrag D, Kibel AS, Freedman ML, The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer, Cancer 123 (2017) 3532–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS, Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers, N. Engl. J. Med 361 (2009) 123–134. [DOI] [PubMed] [Google Scholar]
- [74].Petrovics G, Price DK, Lou H, Chen Y, Garland L, Bass S, Jones K, Kohaar I, Ali A, Ravindranath L, Young D, Cullen J, Dorsey TH, Sesterhenn IA, Brassell SA, Rosner IL, Ross D, Dahut W, Ambs S, Figg WD, Srivastava S, Dean M, Increased frequency of germline BRCA2 mutations associates with prostate cancer metastasis in a racially diverse patient population, Prostate Cancer Prostatic Dis. 22 (2019) 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Beebe-Dimmer JL, Zuhlke KA, Johnson AM, Liesman D, Cooney KA, Rare germline mutations in African American men diagnosed with early-onset prostate cancer, Prostate 78 (2018) 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yadav S, Anbalagan M, Baddoo M, Chellamuthu VK, Mukhopadhyay S, Woods C, Jiang W, Moroz K, Flemington EK, Makridakis N, Somatic mutations in the DNA repairome in prostate cancers in African Americans and Caucasians, Oncogene 39 (2020) 4299–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Rayford W, Beksac AT, Alger J, Alshalalfa M, Ahmed M, Khan I, Falagario UG, Liu Y, Davicioni E, Spratt DE, Schaeffer EM, Feng FY, Mahal B, Nguyen PL, Den RB, Greenberger MD, Bradley R, Watson JM, Beamer M, Stamatakis L, Carmen DJ, Awasthi S, Hwang J, Weil R, Merisaari H, Mohamed N, Deane LA, Chakravarty D, Yadav KK, Yamoah K, Nair SS, Tewari AK, Comparative analysis of 1152 African-American and European-American men with prostate cancer identifies distinct genomic and immunological differences, Commun. Biol 4 (2021) 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Fouquerel E, Lormand J, Bose A, Lee HT, Kim GS, Li J, Sobol RW, Freudenthal BD, Myong S, Opresko PL, Oxidative guanine base damage regulates human telomerase activity, Nat. Struct. Mol. Biol 23 (2016) 1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hewitt G, Jurk D, Marques FD, Correia-Melo C, Hardy T, Gackowska A, Anderson R, Taschuk M, Mann J, Passos JF, Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence, Nat. Commun 3 (2012) 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Xu J, Chang WS, Tsai CW, Bau DT, Xu Y, Davis JW, Thompson TC, Logothetis CJ, Gu J, Leukocyte telomere length is associated with aggressive prostate cancer in localized prostate cancer patients, EBioMedicine 52 (2020) 102616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Heaphy CM, Joshu CE, Barber JR, Davis C, Zarinshenas R, De Marzo AM, Lotan TL, Sfanos KS, Meeker AK, Platz EA, Racial difference in prostate cancer cell telomere lengths in men with higher grade prostate cancer: a clue to the racial disparity in prostate cancer outcomes, Cancer Epidemiol. Biomarkers Prev 29 (2020) 676–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Tang D, Kryvenko ON, Wang Y, Jankowski M, Trudeau S, Rundle A, Rybicki BA, Elevated polycyclic aromatic hydrocarbon-DNA adducts in benign prostate and risk of prostate cancer in African Americans, Carcinogenesis 34 (2013) 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Aronson KJ, Siemiatycki J, Dewar R, Gerin M, Occupational risk factors for prostate cancer: results from a case-control study in Montreal, Quebec, Canada, Am. J. Epidemiol 143 (1996) 363–373. [DOI] [PubMed] [Google Scholar]
- [84].Nock NL, Bock C, Neslund-Dudas C, Beebe-Dimmer J, Rundle A, Tang D, Jankowski M, Rybicki BA, Polymorphisms in glutathione S-transferase genes increase risk of prostate cancer biochemical recurrence differentially by ethnicity and disease severity, Cancer Causes Control 20 (2009) 1915–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Barrington WE, Schenk JM, Etzioni R, Arnold KB, Neuhouser ML, Thompson IM Jr., Lucia MS, Kristal AR, Difference in association of obesity with prostate cancer risk between US African American and non-hispanic white men in the selenium and vitamin E cancer prevention trial (SELECT), JAMA Oncol. 1 (2015) 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, Cheng L, Masterson TA, Liu X, Ratliff TL, Cheng JX, Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness, Cell Metabol. 19 (2014) 393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Amling CL, Riffenburgh RH, Sun L, Moul JW, Lance RS, Kusuda L, Sexton WJ, Soderdahl DW, Donahue TF, Foley JP, Chung AK, McLeod DG, Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy, J. Clin. Oncol 22 (2004) 439–445. [DOI] [PubMed] [Google Scholar]
- [88].Beebe-Dimmer JL, Nock NL, Neslund-Dudas C, Rundle A, Bock CH, Tang D, Jankowski M, Rybicki BA, Racial differences in risk of prostate cancer associated with metabolic syndrome, Urology 74 (2009) 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhou X, Mei H, Agee J, Brown T, Mao J, Racial differences in distribution of fatty acids in prostate cancer and benign prostatic tissues, Lipids Health Dis. 18 (2019) 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Bader DA, McGuire SE, Tumour metabolism and its unique properties in prostate adenocarcinoma, Nat. Rev. Urol 17 (2020) 214–231. [DOI] [PubMed] [Google Scholar]
- [91].Wallace TA, Prueitt RL, Yi M, Howe TM, Gillespie JW, Yfantis HG, Stephens RM, Caporaso NE, Loffredo CA, Ambs S, Tumor immunobiological differences in prostate cancer between African-American and European-American men, Cancer Res. 68 (2008) 927–936. [DOI] [PubMed] [Google Scholar]
- [92].Swinnen JV, Vanderhoydonc F, Elgamal AA, Eelen M, Vercaeren I, Joniau S, Van Poppel H, Baert L, Goossens K, Heyns W, Verhoeven G, Selective activation of the fatty acid synthesis pathway in human prostate cancer, Int. J. Cancer 88 (2000) 176–179. [DOI] [PubMed] [Google Scholar]
- [93].Wu X, Daniels G, Lee P, Monaco ME, Lipid metabolism in prostate cancer, Am J Clin Exp Urol 2 (2014) 111–120. [PMC free article] [PubMed] [Google Scholar]
- [94].Huang WC, Li X, Liu J, Lin J, Chung LW, Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells, Mol. Cancer Res 10 (2012) 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhang L, Wang J, Wang Y, Zhang Y, Castro P, Shao L, Sreekumar A, Putluri N, Guha N, Deepak S, Padmanaban A, Creighton CJ, Ittmann M, MNX1 is oncogenically upregulated in African-American prostate cancer, Cancer Res. 76 (2016) 6290–6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bitting RL, Armstrong AJ, Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer, Endocr. Relat. Cancer 20 (2013) R83–R99. [DOI] [PubMed] [Google Scholar]
- [97].Jiao J, Wang S, Qiao R, Vivanco I, Watson PA, Sawyers CL, Wu H, Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development, Cancer Res. 67 (2007) 6083–6091. [DOI] [PubMed] [Google Scholar]
- [98].Wang BD, Ceniccola K, Hwang S, Andrawis R, Horvath A, Freedman JA, Olender J, Knapp S, Ching T, Garmire L, Patel V, Garcia-Blanco MA, Patierno SR, Lee NH, Alternative splicing promotes tumour aggressiveness and drug resistance in African American prostate cancer, Nat. Commun 8 (2017) 15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wang Y, Wang J, Zhang L, Karatas OF, Shao L, Zhang Y, Castro P, Creighton CJ, Ittmann M, RGS12 is a novel tumor-suppressor gene in African American prostate cancer that represses AKT and MNX1 expression, Cancer Res. 77 (2017) 4247–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Zadra G, Photopoulos C, Tyekucheva S, Heidari P, Weng QP, Fedele G, Liu H, Scaglia N, Priolo C, Sicinska E, Mahmood U, Signoretti S, Birnberg N, Loda M, A novel direct activator of AMPK inhibits prostate cancer growth by blocking lipogenesis, EMBO Mol. Med 6 (2014) 519–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zadra G, Ribeiro CF, Chetta P, Ho Y, Cacciatore S, Gao X, Syamala S, Bango C, Photopoulos C, Huang Y, Tyekucheva S, Bastos DC, Tchaicha J, Lawney B, Uo T, D’Anello L, Csibi A, Kalekar R, Larimer B, Ellis L, Butler LM, Morrissey C, McGovern K, Palombella VJ, Kutok JL, Mahmood U, Bosari S, Adams J, Peluso S, Dehm SM, Plymate SR, Loda M, Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer, Proc. Natl. Acad. Sci. U. S. A 116 (2019) 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Gillard M, Javier R, Ji Y, Zheng SL, Xu J, Brendler CB, Crawford SE, Pierce BL, Griend DJV, Franco OE, Elevation of stromal-derived mediators of inflammation promote prostate cancer progression in African-American men, Cancer Res. 78 (2018) 6134–6145. [DOI] [PubMed] [Google Scholar]
- [103].Powell IJ, Dyson G, Land S, Ruterbusch J, Bock CH, Lenk S, Herawi M, Everson R, Giroux CN, Schwartz AG, Bollig-Fischer A, Genes associated with prostate cancer are differentially expressed in African American and European American men, Cancer Epidemiol. Biomarkers Prev 22 (2013) 891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].King Thomas J, Mir H, Kapur N, Singh S, Racial differences in immunological landscape modifiers contributing to disparity in prostate cancer, Cancers (Basel) (2019) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Furusato B, Mohamed A, Uhlen M, Rhim JS, CXCR4 and cancer, Pathol. Int 60 (2010) 497–505. [DOI] [PubMed] [Google Scholar]
- [106].Powell IJ, Chinni SR, Reddy SS, Zaslavsky A, Gavande N, Pro-inflammatory cytokines and chemokines initiate multiple prostate cancer biologic pathways of cellular proliferation, heterogeneity and metastasis in a racially diverse population and underlie the genetic/biologic mechanism of racial disparity: Update, Urol. Oncol 39 (2021) 34–40. [DOI] [PubMed] [Google Scholar]
- [107].Sartor O, Armstrong AJ, Ahaghotu C, McLeod DG, Cooperberg MR, Penson DF, Kantoff PW, Vogelzang NJ, Hussain A, Pieczonka CM, Shore ND, Quinn DI, Small EJ, Heath EI, Tutrone RF, Schellhammer PF, Harmon M, Chang NN, Sheikh NA, Brown B, Freedland SJ, Higano CS, Survival of African-American and Caucasian men after sipuleucel-T immunotherapy: outcomes from the PROCEED registry, Prostate Cancer Prostatic Dis. 23 (2020) 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hawley JE, Pan S, Kandadi H, Chaimowitz MG, Sheikh N, Drake CG, Analysis of Circulating Immune Biomarkers by Race in Men with Metastatic Castration-Resistant Prostate Cancer Treated with Sipuleucel-T, JNCI, Journal of the National Cancer Institute, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ciardiello D, Elez E, Tabernero J, Seoane J, Clinical development of therapies targeting TGFβ: current knowledge and future perspectives, Ann. Oncol 31 (2020) 1336–1349. [DOI] [PubMed] [Google Scholar]
- [110].Navone NM, van Weerden WM, Vessella RL, Williams ED, Wang Y, Isaacs JT, Nguyen HM, Culig Z, van der Pluijm G, Rentsch CA, Marques RB, de Ridder CMA, Bubendorf L, Thalmann GN, Brennen WN, Santer FR, Moser PL, Shepherd P, Efstathiou E, Xue H, Lin D, Buijs J, Bosse T, Collins A, Maitland N, Buzza M, Kouspou M, Achtman A, Taylor RA, Risbridger G, Corey E, Movember GAP1 PDX project: an international collection of serially transplantable prostate cancer patient-derived xenograft (PDX) models, Prostate 78 (2018) 1262–1282. [DOI] [PubMed] [Google Scholar]
- [111].Forde AT, Crookes DM, Suglia SF, Demmer RT, The weathering hypothesis as an explanation for racial disparities in health: a systematic review, Ann. Epidemiol 33 (2019) 1–18 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hudson DL, Neighbors HW, Geronimus AT, Jackson JS, Racial discrimination, John henryism, and depression among African Americans, J. Black Psychol 42 (2016) 221–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Coker AL, Sanderson M, Ellison GL, Fadden MK, Stress, coping, social support, and prostate cancer risk among older African American and Caucasian men, Ethn. Dis 16 (2006) 978–987. [PubMed] [Google Scholar]
- [114].Castagné R, Kelly-Irving M, Campanella G, Guida F, Krogh V, Palli D, Panico S, Sacerdote C, Tumino R, Kleinjans J, de Kok T, Kyrtopoulos SA, Lang T, Stringhini S, Vermeulen R, Vineis P, Delpierre C, Chadeau-Hyam M, Biological marks of early-life socioeconomic experience is detected in the adult inflammatory transcriptome, Sci. Rep 6 (2016) 38705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Reuben A, Sugden K, Arseneault L, Corcoran DL, Danese A, Fisher HL, Moffitt TE, Newbury JB, Odgers C, Prinz J, Rasmussen LJH, Williams B, Mill J, Caspi A, Association of neighborhood disadvantage in childhood with DNA methylation in young adulthood, JAMA Netw. Open 3 (2020), e206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Fiorito G, Polidoro S, Dugúe PA, Kivimaki M, Ponzi E, Matullo G, Guarrera S, Assumma MB, Georgiadis P, Kyrtopoulos SA, Krogh V, Palli D, Panico S, Sacerdote C, Tumino R, Chadeau-Hyam M, Stringhini S, Severi G, Hodge AM, Giles GG, Marioni R, Karlsson Linńer R, O’Halloran AM, Kenny RA, Layte R, Baglietto L, Robinson O, McCrory C, Milne RL, Vineis P, Social adversity and epigenetic aging: a multi-cohort study on socioeconomic differences in peripheral blood DNA methylation, Sci. Rep 7 (2017) 16266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Gomez SL, Shariff-Marco S, DeRouen M, Keegan TH, Yen IH, Mujahid M, Satariano WA, Glaser SL, The impact of neighborhood social and built environment factors across the cancer continuum: current research, methodological considerations, and future directions, Cancer 121 (2015) 2314–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]


