Abstract
Surgical site infections (SSIs) constitute a major problem among patients who undergo surgery for oral cancer and remain a substantial cause of morbidity, prolonged hospitalization and death. The aim of this study was to assess the microbial spectrum of post-operative wound infections and to assess the outcome of appropriate antibiotic usage in patients who underwent surgery for oral cancer. This was a prospective observational study done in a tertiary care cancer hospital. Patients who underwent surgery for cancers of the oral cavity during the period January 2014 to December 2016 were included in the study. The spectrum of infections, risk factors, microbial profile, antibiotic susceptibility pattern, treatment given and outcome were assessed. A total of 1431 patients who underwent surgery for cancers of the oral cavity during the study period were followed up. SSIs were noticed in 118 (8%) post-operative cases. This included 55 (76.4%) incisional SSIs and 17 (23.6%) organ/space SSIs. Culture of the surgical site in 72 infected patients yielded a total of 122 isolates. Thirty patients (41.6%) had polymicrobial infections. Gram-negative bacterial isolates (70.5%) outnumbered gram-positive bacterial isolates (27%). Majority of the patients (48 patients—66.6%) were in stage IV disease. Successful management of patients with bacterial infections depends on early identification of bacterial pathogens and selection of an effective antibiotic against the organism. Judicial use of antibiotics is also very essential to prevent the development of drug resistance.
Keywords: Surgical site infection, Oral cavity cancer, Gram-negative bacteria, Antibiotics
Introduction
Surgical site infections (SSIs) remain one of the serious post-operative complications, occurring in nearly 2% of surgical procedures, constituting approximately 20% of all of health care-associated infections [1].
The frequency of this complication is increased in oral cavity cancer patients compared to surgeries in other anatomical sites because of surgical site exposure to bacteria and the need to recreate the mucosal barrier of the oral cavity following surgery, particularly when there is a communication into the neck.
SSIs following head and neck cancer surgery may occur in as many as 10–45% of cases despite antibiotic prophylaxis [2]. The development of SSIs can further lead to serious complications including wound breakdown, mucocutaneous fistulae and sepsis. Delayed wound healing may also result in poor cosmetic outcomes, delayed oral intake and delay in adjuvant therapy [2].
The risk factors for infections include long duration of surgery, co-morbid conditions like diabetes, elderly patients, overweight, smoking, malignancy, emergency surgery and weakened immune system.
SSIs have been defined by the Centre for Disease Control and Prevention as infections within the first 30 postoperative days with at least one of several factors including purulent drainage, positive culture and either a deliberate incision and drainage or presence of signs and symptoms [3].
Even though SSI is a relatively serious problem, there are only very few published reports about the bacterial pathogens and their antibiogram involved in SSIs in cancer patients. Data from our study could help the clinicians to make decisions on issues of infection control pertaining to surgical wound sepsis and also to use the antibiotics judiciously.
The present study was conducted in a tertiary care cancer centre to assess the pattern of post-operative wound infection among patients who underwent surgery for oral cavity cancers and also identify the causative organisms and determine their susceptibility profile.
The purpose of the study is to assess the pattern of post-operative wound infections among head and neck cancer patients and also to determine the pathogens associated with SSIs and their susceptibility profile.
Methodology
All patients who underwent surgery for cancers of the oral cavity during the period January 2014 to December 2016 were included in the study. Those who were referred for surgery after receiving chemotherapy or radiotherapy and below 18 years were excluded from the study.
The patients were admitted on the day before surgery after pre anaesthetic check-up. Prophylactic antibiotics (2nd generation cephalosporin along with metronidazole) were given 30 min before surgery.
During the post-operative period, the antibiotics were given depending on the stage and co morbid conditions and the oral sips started as soon as the patients could swallow freely. The Ryles tube was retained for feeding for those patients who could not swallow. After removing the Ryles tube the patients were observed for one more day to teach them regarding diet, oral cleanliness and wound care. The duration of the antibiotic coverage varied between 2 and 5 days.
Usually the patients were discharged on the 4th or 5th post-operative day, but if there were any signs and symptoms of SSI, they were retained in the ward, pus swabs or aspirates were collected from infected surgical sites and processed in microbiology laboratory as per standard protocols. Antibiotics were either started or changed as per culture and sensitivity reports. If wound infection persisted, or if there was deterioration of clinical condition, repeat culture was done and appropriate treatment given.
Data Collection
Prospective data collection was done with the help of a questionnaire for SSIs which included demographic details, risk factors, details of surgery, pattern of post-operative wound infections, the causative agents, susceptibility profile, treatment given and outcome.
Microbiological Methods
Pus swabs were collected from infected surgical sites suspected of SSI and transported to the laboratory in Amies transport medium. Aspirated samples were also sent in some cases. Culture was done using standard bacteriological procedures. Bacterial identification was done depending on the colony morphology, gram stain and routine biochemical tests. Susceptibility testing was done using the disk diffusion technique according to Clinical and Laboratory Standards Institute (CLSI) guidelines.
Results
Patient Characteristics
A total of 1431 patients underwent surgery for cancers of the oral cavity during the period and were followed up for an average of 23.2 months. The mean age of the study group was 59.3 years. SSIs were noticed in 118 (8%) post-operative cases. 72 (61%) patients had positive culture results (males 40 and females 32). 55 patients (76.4%) had incisional SSIs and 17 (23.6%) patients had organ or space SSIs.
Majority of the patients were in stage IV—48 patients (66.6%) and 14 patients (19.4%) were in stage III of disease (Fig. 1). 32 patients (44.4%) did not have any co-morbidities, 19 patients (26.4%) had diabetes mellitus, 10 patients (13.8%) had hypertension and 9 patients (12.5%) had both diabetes and hypertension (Fig. 2). Duration of surgery was less than three hours in 13 cases, but more than three hours in 59 cases.
Fig. 1.
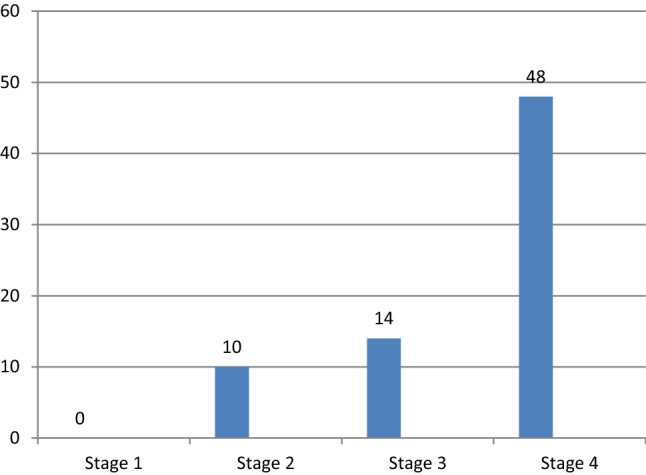
Stage-wise distribution of patients with surgical site infection
Fig. 2.

Co-morbidities of patients with surgical skin infections
The discharge from surgical site was the most common mode of presentation in case of infections followed by wound dehiscence. 44 patients (61.1%) had discharge from the wound site and 19 patients (26.4%) had wound dehiscence. Another mode of presentation was discoloration of the skin and increased temperature at the surgical site. In majority of cases the signs related to wound infection were noticed within first 5 days of surgery. In seven patients the infection was noticed during the first follow up i.e. after 2 weeks.
Microbial Profile
Culture of the surgical site in 72 infected patients yielded a total of 122 isolates (Table 1). Thirty patients (41.6%) had polymicrobial infections. Gram-negative bacterial isolates (70.5%) outnumbered Gram-positive bacterial isolates (27%). Candida species were isolated from three patients (2.5%). Most frequently isolated pathogens were Klebsiella species (19.6%) and Pseudomonas aeruginosa (19.6%) followed by Staphylococcus aureus (15.6%) and E. coli (14.7%).
Table 1.
Distribution of major microorganisms causing surgical site infection (n = 122)
| Microorganism | No. (%) |
|---|---|
| Gram-positive pathogens | 33 (27) |
| Staphylococcus aureus | 19 (15.6) |
| Non diphtheritic Corynebacterium species | 6 (4.9) |
| Enterococci | 4 (3.2) |
| Coagulase-negative Staphylococcus | 2 (1.6) |
| Alpha hemolytic Streptococci | 2 (1.6) |
| Gram-negative pathogens | 86 (70.5) |
| Klebsiella pneumoniae | 24 (19.6) |
| Pseudomonas aeruginosa | 24 (19.6) |
| E. coli | 18 (14.7) |
| Enterobacter species | 8 (6.5) |
| Morganella morganii | 6 (4.9) |
| Acinetobacter species | 6 (4.9) |
| Candida species | 3 (2.5) |
Anti-Microbial Susceptibility
47.6% of enterobacterial isolates (Klebsiella species and E.coli) were found to be extended spectrum beta-lactamase (ESBL) producers. (ie. resistant to most beta-lactam antibiotics, including penicillins, cephalosporins, and aztreonam). Four of the S. aureus isolates (21%) were methicillin-resistant (MRSA).
More than 90% of the gram-negative bacterial isolates were susceptible to imipenem, meropenem and amikacin. High level resistance was noticed for cephalosporins, aztreonam and quinolones (ciprofloxacin and levofloxacin). All gram-positive isolates were susceptible to vancomycin and linezolid. The susceptibility pattern of the gram-negative and gram-positive bacteria which were more frequently isolated is given in Tables 2 and 3.
Table 2.
Antibiotic susceptibility pattern of the common gram-negative bacterial isolates
| Antibiotics tested | Klebsiella species and E. coli (n = 74) | P. aeruginosa (n = 24) | Acinetobacter sp. (n = 6) |
|---|---|---|---|
| % Susceptible | % Susceptible | % Susceptible | |
| Imipenem | 98.7 | 100 | 83.3 |
| Meropenem | 90.6 | 100 | 83.3 |
| Amikacin | 87.9 | 100 | 66.7 |
| Piperacillin/tazobactam | 54.1 | 95.8 | 33.4 |
| Cefoperazone/sulbactam | 52.8 | 95.8 | 66.7 |
| Gentamicin | 43.2 | 100 | 50 |
| Cefepime | 14.9 | 95.8 | 50 |
| Ceftazidime | 13.6 | 95.8 | 33.4 |
| Ciprofloxacin | 32.5 | 87.5 | 16.7 |
| Levofloxacin | 43.2 | 87.5 | 16.7 |
| Doxycycline | 36.5 | 83.3 | |
| Cotrimoxazole | 36.5 | 33.4 | |
| Aztreonam | 13.6 | 95.8 | 33.4 |
Table 3.
Antibiotic susceptibility pattern of Staphylococcus aureus
| Antibiotic tested | % Susceptible |
|---|---|
| Vancomycin | 100 |
| Linezolid | 100 |
| Amikacin | 94.8 |
| Doxycycline | 94.8 |
| Cloxacillin | 78.9 |
| Cefazolin | 78.9 |
| Amoxicillin/clavulanic acid | 78.9 |
| Piperacillin/tazobactam | 78.9 |
| Imipenem | 78.9 |
| Meropenem | 78.9 |
| Cotrimoxazole | 73.7 |
| Erythromycin | 52.7 |
| Levofloxacin | 31.6 |
| Ciprofloxacin | 15.8 |
| Penicillin G | 5.3 |
Treatment
The staphylococcal infections in most cases were controlled with cefazolin, amoxicillin/clavulanic acid and aminoglycosides. MRSA isolates responded to vancomycin. The gram-negative infections caused by Pseudomonas and Klebsiella species responded to cefoperazone/sulbactam or piperacillin/tazobactam and amikacin combination. Those cases which did not respond the cefoperazone/sulbactam or combination therapy were treated with imipenem.
Outcome
On administration of appropriate antimicrobial therapy, after ensuring no allergic reactions the symptoms subsided in all patients. Some patients were referred to local hospital for continuation of the treatment. Others were discharged after completion of the antibiotic therapy from our centre. All patients were asked to report at the outpatient clinic 7 days after the completion of the treatment. They were evaluated further for any symptoms. But repetition of culture was not done in any of the patients. No adverse events were reported during the treatment, antibiotics once started were never changed in any of the patients.
Discussion
In hospitalized patients, SSIs are the third most frequently reported infection and often account for 12–16% of all nosocomial infections [4]. Successful management of patients with bacterial infections depends on early identification of bacterial pathogens and selection of an effective antibiotic against the organism. Judicial use of antibiotics is very essential to prevent the development of drug resistant organisms.
The incidence of SSI in the present study was found to be 8%. Durand et al. conducted a study to determine the time course and microbiology of SSIs after head and neck free flap surgery which showed an SSI incidence of 13.3% [5]. Wound cultures were polymicrobial, gram-negative bacilli (44% of cases), MRSA (20%), and methicillin-sensitive Staphylococcus aureus (MSSA) (16%). In our study too, gram-negative organisms predominated which constituted about 70.5% of all isolates. Similarly in another study by Goyal et al. to evaluate SSI in major head and neck surgeries involving pedicled flap reconstruction, it was seen that SSIs occurred in 9.1% and were associated with a longer length of stay (P value = 0.004) but with no particular risk factors [6].
All patients ≥ 18 years who underwent oral cavity resection and neck dissection for squamous cell carcinoma, requiring either a mandibulotomy or mandibulectomy with free flap reconstruction and osseous plating performed at the University Health Network in Toronto, Canada between 1997 and 2014 were identified and followed up by Christopher et al. and they found that 84 patients (23.0%) developed SSIs within 30 days of their operation. The most common SSI formed were neck abscesses (11.5%), and oro-cutaneous fistulae (10%) [2].
The surgeries in the present study were clean-contaminated and involved surgical opening of the mucosa in the oral cavity. As per the National Healthcare Safety Network (NHSN) guidelines 2017, SSIs have been defined as infection within the first 30–90 postoperative days depending on the procedure with at least one of several factors, including purulent drainage, positive culture, and either a deliberate incision and drainage or presence of supporting signs and symptoms.
Surgical site infection is not an uncommon phenomenon and the types of infection include superficial incisional SSI, deep incisional SSI, and organ or space SSI. The superficial incisional SSI is confined to the skin where the incision was made. The deep incisional infection occurs beneath the incisional area in the fascia, muscle and the tissue surrounding the muscle. When infection occurs in area other than the skin, fascia and muscle it is called organ or space SSI which occurs in the organ or in the space between the organs.
In head and neck surgeries, most of the infections are confined to skin, muscle, fascia and soft tissues surrounding it. It is said that the extent of microbial contamination at the primary surgical site is an important risk factor for subsequent wound infections. A SSI occurs when micro-organisms get into the part of the body that has been operated on and multiply in the tissues [7].
Multiple studies indicate that the most common types of adverse events affecting hospitalized patients are adverse drug events, Hospital Care Associated Infections (HCAI), and surgical complications [8].
The degree of SSI is linked to the type of surgical wound. In clean wounds where there is no visible infection at the time of surgery, the risk of infection is less. But in wounds where there is spillage of contents of the organ into the wound, the risk of infection is varies between 13 and 20%. In case of surgeries of oral cavity there is always a risk of contamination of wound by saliva. Poor oral hygiene is also an important factor for the development of wound complications during the post-operative period as evidenced in a prospective study of 186 head and neck cancer patients [9]. According to Gourin et al., patients are twice more likely to have an SSI, dehiscence and fistula if they experienced perioperative weight loss [10].
Around 40–60% of SSIs are preventable and effective control of SSIs relies on a multitude of interventions like surveillance, antimicrobial prophylaxis, eradication of carrier status, infection control programmes and education. In cases where there is wound dehiscence, secondary suturing was never attempted, but dressings and application of topical agents helped healing by secondary intention [11].
The factors responsible for the SSIs include patient factors like age, co-morbidities, nutritional status, prophylactic antibiotics, duration of surgery etc. [12]. In our study we found that the risk of infection is high when the duration of surgery exceeds 3 h. The incidence of gram-negative infection was more than gram positive infection, the incidence of mixed infection was higher than the gram-positive infections alone. The risk of infection is more in patients with diabetes, compared to patients with hypertension. Similarly risk of infection is more in patients who were in advanced stages of the disease. In majority of cases the infection was superficial in nature compared to deep space infection.
Within the realms of SSI there are pertinent Cochrane reviews to evaluate the six tenets central to conventional surgical practice:
Preoperative skin antiseptics for preventing SSIs after clean surgery [13]
Antimicrobial drugs for treating MRSA colonisation [14]
Preoperative hair removal to reduce SSI [15]
Surgical hand antisepsis to reduce SSIs [16]
Preoperative bathing or showering with skin antiseptics to prevent SSI [17]
Dressings and topical agents for surgical wound healing by secondary intention [11].
A survey conducted in 183 US hospitals with 11,282 patients reported that 4% of patients had at least one HCAI with the most common microorganism being Clostridium difficile. Most infections were SSIs, pneumonia, and gastrointestinal infections [18]. The movement and number of staff and the structural features of the operating theatre also affect the incidence of SSIs [19]. Jacobson et al. in their review of literature described that radiotherapy delay the wound healing by affecting the normal as well as tumour cells [20]. Shamberger et al. in their article says chemotherapy affects the inflammatory phase delaying the wound healing [21]. We did not include those who received radiotherapy and chemotherapy prior to surgery in the study since they are known factors for delayed wound healing.
Odom-Forren says that 40–60% of SSIs are preventable and that effective control of SSIs relies on a multitude of interventions that includes surveillance, antimicrobial prophylaxis, eradication of carrier status, infection control programmes and education [22].
Conclusions
In the present study, it was noticed that prolonged duration of surgery, diabetes, advanced stage of disease are the risk factors for SSIs. Gram-negative organisms are more common than gram positive organisms causing infections. Majority of infections are superficial infections and can be controlled by prophylactic antibiotics. Klebsiella pneumoniae is the most common gram-negative organism followed by P. aeruginosa. The most common gram-positive organism isolated was S. aureus. The majority of gram-negative infections were susceptible to imipenem, meropenem and amikacin. The mixed infections in most of the cases responded to amino glycosides.
Acknowledgements
We take this opportunity to thank the faculty, fellows, post graduates and staff of the department of Head and Neck surgery and Microbiology for their whole-hearted cooperation in completion of this study.
Compliance with Ethical Standards
Conflict of interest
There is no conflict of interest pertaining to this study.
Ethical Approval
All investigations and procedures done in studies were in accordance with the ethical standards of Regional Cancer Centre, Thiruvananthapuram approved by the Institutional Ethics Committee.
Ethical Statement
There was adherence to ethical standards in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gregory L, Kathy F, Valerie H, et al. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387–397. doi: 10.1016/j.ajic.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Yao CM, Ziai H, Tsang G, et al. Surgical site infections following oral cavity cancer resection and reconstruction is a risk factor for plate exposure. J Otolaryngol Head Neck Surg. 2017;46:30. doi: 10.1186/s40463-017-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992;20(5):271–274. doi: 10.1016/S0196-6553(05)80201-9. [DOI] [PubMed] [Google Scholar]
- 4.Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6:428–442. doi: 10.1128/CMR.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durand ML, Yarlagadda BB, Rich DL, et al. The time course and microbiology of surgical site infections after head and neck free flap surgery. Laryngoscope. 2015;125(5):1084–1089. doi: 10.1002/lary.25038. [DOI] [PubMed] [Google Scholar]
- 6.Goyal N, Yarlagadda BB, Deschler DG, et al. Surgical site infections in major head and neck surgeries involving pedicled flap reconstruction. Ann Otol Rhinol Laryngol. 2017;126:20–28. doi: 10.1177/0003489416672871. [DOI] [PubMed] [Google Scholar]
- 7.Berard F, Gandon J. Postoperative wound infections: the influence of ultraviolet irradiation of the operating room and of various other factors. Ann Surg. 1964;160:1–192. [PubMed] [Google Scholar]
- 8.Haque M, Sartelli M, McKimm J, et al. Health care-associated infections: an overview. Infect Drug Resist. 2018;11:2321–2333. doi: 10.2147/IDR.S177247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaukar DA, Deshmukh AD, Majeed T, et al. Factors affecting wound complications in head and neck surgery: a prospective study. Indian J Med Paediatr Oncol. 2013;34:247–251. doi: 10.4103/0971-5851.125236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gourin CG, Starmer HM, Herbert RJ, et al. Short- and long-term outcomes of laryngeal cancer care in the elderly. Laryngoscope. 2015;125:924–933. doi: 10.1002/lary.25012. [DOI] [PubMed] [Google Scholar]
- 11.Vermeulen H, Ubbink D, Goossens A, et al. Dressings and topical agents for surgical wounds healing by secondary intention. Cochrane Database Syst Rev. 2004;2:CD003554. doi: 10.1002/14651858.CD003554.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Culver DH, Horan TC, Gaynes RP, et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am J Med. 1991;91:152S–157S. doi: 10.1016/0002-9343(91)90361-Z. [DOI] [PubMed] [Google Scholar]
- 13.Edwards PS, Lipp A, Holmes A. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev. 2004;3:CD003949. doi: 10.1002/14651858.CD003949.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Loeb MB, Main C, Eady A, et al. Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization. Cochrane Database Syst Rev. 2003;4:CD003340. doi: 10.1002/14651858.CD003340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanner J, Norrie P, Melen K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2011;11:CD004122. doi: 10.1002/14651858.CD004122.pub4. [DOI] [PubMed] [Google Scholar]
- 16.Tanner J, Swarbrook S, Stuart J. Surgical hand antisepsis to reduce surgical site infection. Cochrane Database Syst Rev. 2008;1:CD004288. doi: 10.1002/14651858.CD004288.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev. 2006;2:CD004985. doi: 10.1002/14651858.CD004985.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spagnolo AM, Ottria G, Amicizia D, et al. Operating theatre quality and prevention of surgical site infections. J Prev Med Hyg. 2013;54(3):131–137. [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobson LK, Johnson MB, Dedhia RD, et al. Impaired wound healing after radiation therapy: a systematic review of pathogenesis and treatment. J Plast Reconstr Aesthet. 2017;13:92–105. [Google Scholar]
- 21.Shamberger RC, Devereux DF, Brennan MF. The effect of chemotherapeutic agents on wound healing. Int Adv Surg Oncol. 1981;4:15–58. [PubMed] [Google Scholar]
- 22.Odom-Forren J. Preventing surgical site infections. Nursing. 2006;36:58–63. doi: 10.1097/00152193-200606000-00045. [DOI] [PubMed] [Google Scholar]


