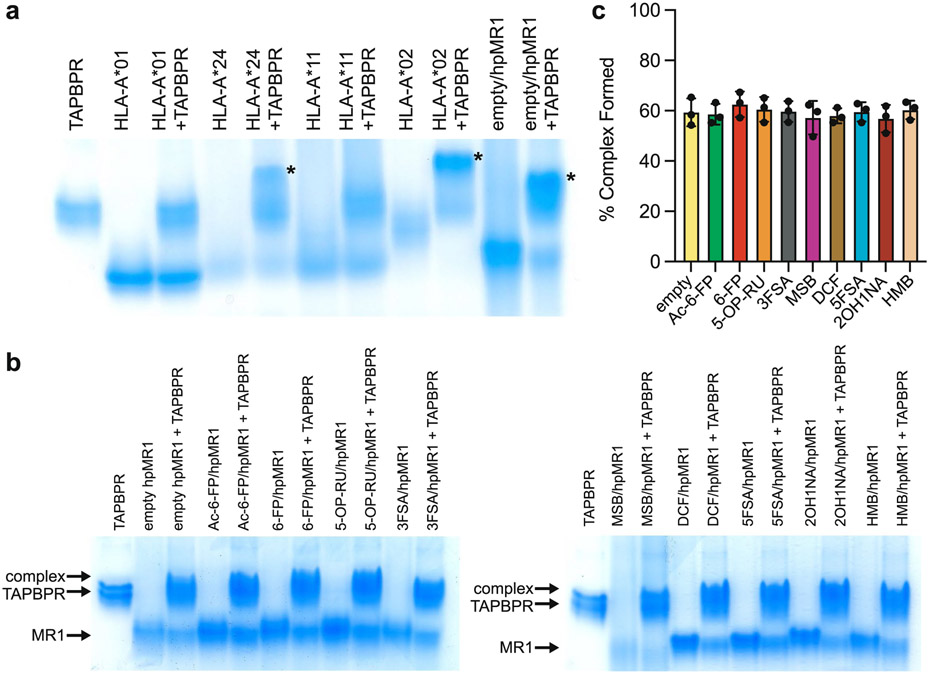
Extended Data Fig. 4 ∣. Ligand independence of the interaction between hpMR1 and TAPBPR by native gel shift assay.
a, Native gel shift analysis for empty hpMR1/bβ2m/TAPBPR and empty MHC-I/TAPBPR complex formation. Each lane is loaded with free TAPBPR, free UVirrad/MHC-I, free empty hpMR1/bβ2m, or 1:1 molar ratio mixture with TAPBPR. Asterisk (*) denote complex formation. b, Native gel shift analysis where each lane is loaded with free TAPBPR, free hpMR1/bβ2m without (empty) or with ligands, or 1:1 mixtures with TAPBPR. Ligand/hpMR1 complexes were prepared by in vitro refolding as described in the main text. c, Quantification of the % of hpMR1/bβ2m/TAPBPR complexes formed in the presence of different ligands, relative to the free MR1 band, based on native gel electrophoresis and band quantification using ImageJ. Data are mean ± SD for n = 3 technical replicates.