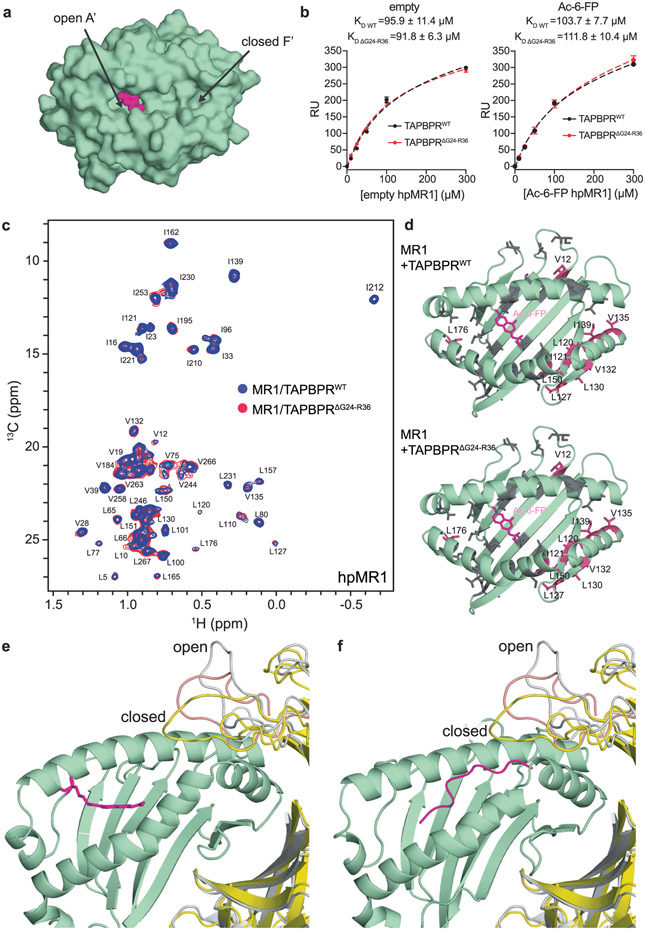
Extended Data Fig. 8 ∣. The TAPBPR G24-R36 loop does not interact with the MR1 groove.
a, Surface representation of the groove of Ac-6-FP loaded MR1 (PDB ID 4PJ5, TCR removed) showing the open A’ pocket and closed F’ pocket. Ac-6-FP is shown as magenta spheres, MR1 is shown as a green surface. b, Plot of response units (RU) from SPR sensorgrams of varying concentrations of empty or Ac-6-FP loaded hpMR1/bβ2m flowed over a streptavidin chip coupled with TAPBPRWT-biotin (black) or TAPBPRΔG24-R36-biotin (red). The dotted lines indicate fits. Data are the mean±SD for n = 3 technical replicates. c, 2D 1H-13C methyl HMQC spectra of 100 μM Ac-6-FP/ILVproS labeled hpMR1/bβ2m in complex with TAPBPRWT (blue) or TAPBPRΔG24-R36 (red) recorded at a 1H field of 600 MHz at 25 °C. d, Comparison of chemical shift perturbation (CSP, ΔδCH3, ppm) analysis for residues of hpMR1 upon complex formation with TAPBPRWT versus TAPBPRΔG24-R36. CSPs plotted onto a RosettaCM model of hpMR1/bβ2m (PDB template 4PJ5, TCR removed). Methyl bearing residues affected for hpMR1 upon binding to TAPBPRWT (top) or TAPBPRΔG24-R36 (bottom) are shown in warm pink and unaffected residues in grey. hpMR1 and Ac-6-FP are colored green and magenta, respectively. e, and f, Structural models of the role of the TAPBPR G24-R36 loop for chaperone interactions with (e) Ac-6-FP/hpMR1 and (f) TAX9/HLA-A*02:01.