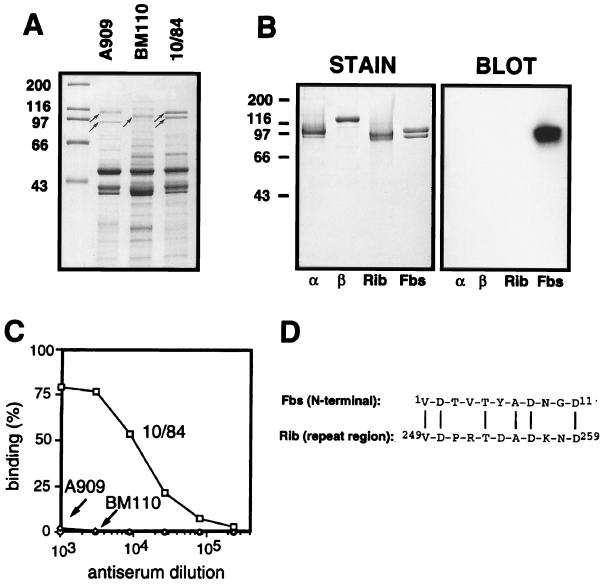
FIG. 1.
Identification of Fbs and characterization of the purified protein. (A) Mutanolysin extracts of group B streptococcal strains analyzed by SDS-PAGE. High-molecular-weight surface proteins appear as distinct bands (arrows). Strains used were A909, a type Ia strain expressing the α (top arrow) and β (bottom arrow) proteins; BM110, a type III strain expressing protein Rib (arrow); and the type V strain 10/84, expressing the protein Fbs (doublet band). (B) Western blot analysis of purified preparations of the α, β, Rib, and Fbs proteins, with rabbit anti-Fbs serum. The antiserum was used at a 1:1,000 dilution, and bound antibodies were detected with 125I-labeled protein G. The autoradiogram was deliberately overexposed to demonstrate the lack of cross-reactivity between Fbs and the α, β, and Rib proteins. In a control blot with preimmune rabbit serum, no signal was obtained. Molecular mass markers for panels A and B are in kilodaltons. (C) Analysis of group B streptococcal strains for cell surface expression of Fbs. The bacteria were analyzed for ability to bind mouse anti-Fbs antibodies, by using 125I-labeled protein A to detect bound antibodies. Strains used were A909 (type Ia), BM110 (type III), and 10/84 (type V). (D) Alignment of the N-terminal amino acid sequence of protein Fbs and an amino acid sequence from the repeat region of protein Rib (34). Vertical lines denote residue identity. The experiments shown here were performed at least twice with similar results.