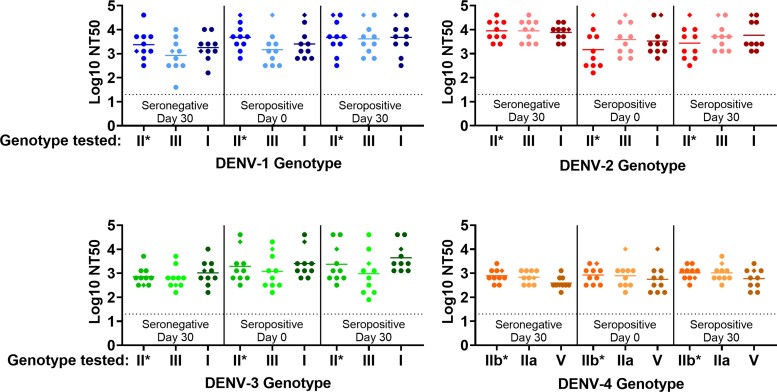
Figure 2.
TAK-003-Elicited neutralizing antibody (nAb) microneutralization test (MNT) titers against homologous tetravalent dengue vaccine (TDV) strains and diverse dengue virus (DENV)-1–4 genotypes in postvaccination serum. DEN-205 antisera from baseline-seronegative (n = 10) and seropositive (n = 10) individuals vaccinated with a single dose of high-dose TDV (n = 18; ● symbols) or TDV (n = 2; ◆ symbols) collected prevaccination and 30 days postvaccination were tested in a MNT against a panel of genetically diverse DENV strains representative of historical (1956–2007) genotypes isolated in Asia and Latin America (Supplementary Table 1). Titers were defined as the serum dilution resulting in a 50% reduction in plaque number compared with virus only control (NT50). Data were truncated at MNT limit of detection (log10 NT50 = 1.3). P values were calculated by ordinary one-way analysis of variance with Sidak’s method for multiple comparisons. Neutralizing titers against nonvaccine genotypes were compared with the homologous vaccine strain within each serum group (naive day 30, preimmune day 0, preimmune day 30) and between serum groups (naive day 30 vs preimmune day 30, preimmune day 0 vs preimmune day 30). No significant differences in NT50 were observed in any of the comparisons. *, Homologous (parental) TDV-1, -2, -3, or -4 strain.