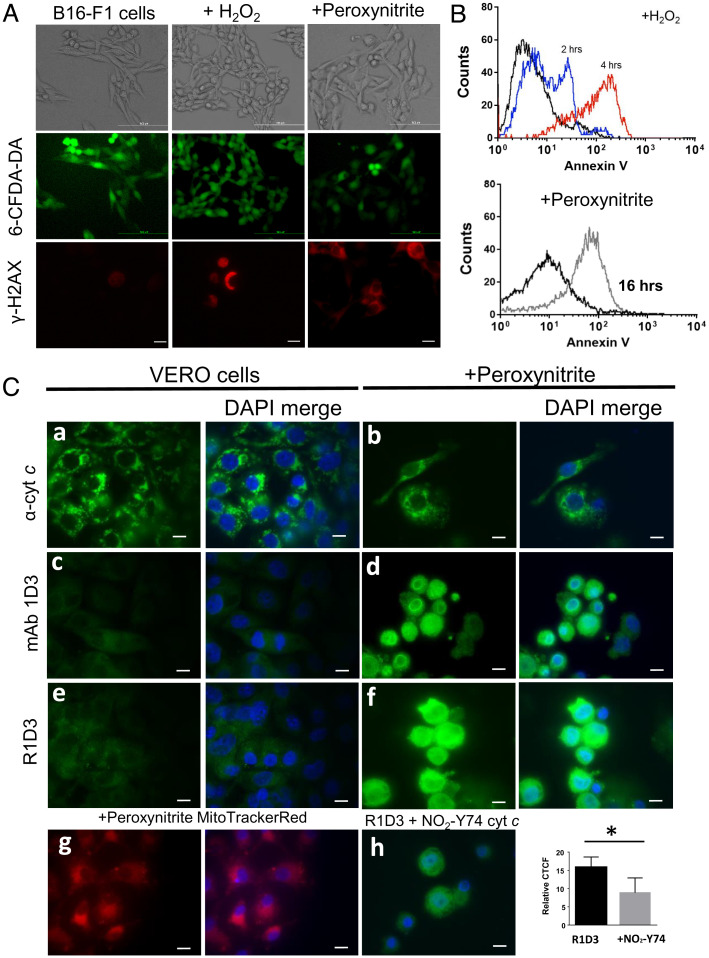
Fig. 5.
Immunocytochemical detection of an alternative conformation of cyt c after peroxynitrite treatment. (A) Plasma membrane integrity and DNA damage. After oxidant treatment (H2O2 or peroxynitrite), B16-F1 living cells were stained with 6CF-DA for 30 min and analyzed by confocal microscopy. Green stain indicates the integrity of the plasma membrane. After oxidant treatment, B16-F1 cells were fixed and probed with antiphosphorylated H2AX antibody followed by antimouse-Cy3 conjugate antibody (red) by confocal microscopy. (B) Phosphatidyl serine exposure was observed using Annexin V-FITC and analyzed by flow cytometry after 2 h (blue histogram) or 4 h (red histogram) for H2O2 (2 mM) (Upper) or peroxynitrite (300 µM) treatment (gray histogram) (Lower). (C) Immunocytochemical detection of an alternative conformation of cyt c. Control conditions and cells treated with peroxynitrite (300 µM in PBS) were incubated for 16 h at 37 °C and 5% CO2. Fixed cells (control or oxidant treated) were probed with antinative cyt c (6H2.B4, a and b), mAb 1D3 (c and d), or mAb R1D3 (e and f) followed by antimouse FITC-labeled conjugated antibody (green) and DAPI (DNA, blue) and then analyzed by confocal microscopy. Mitochondrial distribution of peroxynitrite-treated cells was evaluated with Mito Tracker Red (g). Inhibition of the mAb R1D3 binding was performed by coincubation of the antibody with soluble NO2Y74 cyt c (50 µM, h). Relative CTCF of at least 50 cells in the presence or absence of soluble NO2Y74 were analyzed. * denotes statistical difference P < 0.05. Horizontal scale bars indicate 10 µm.