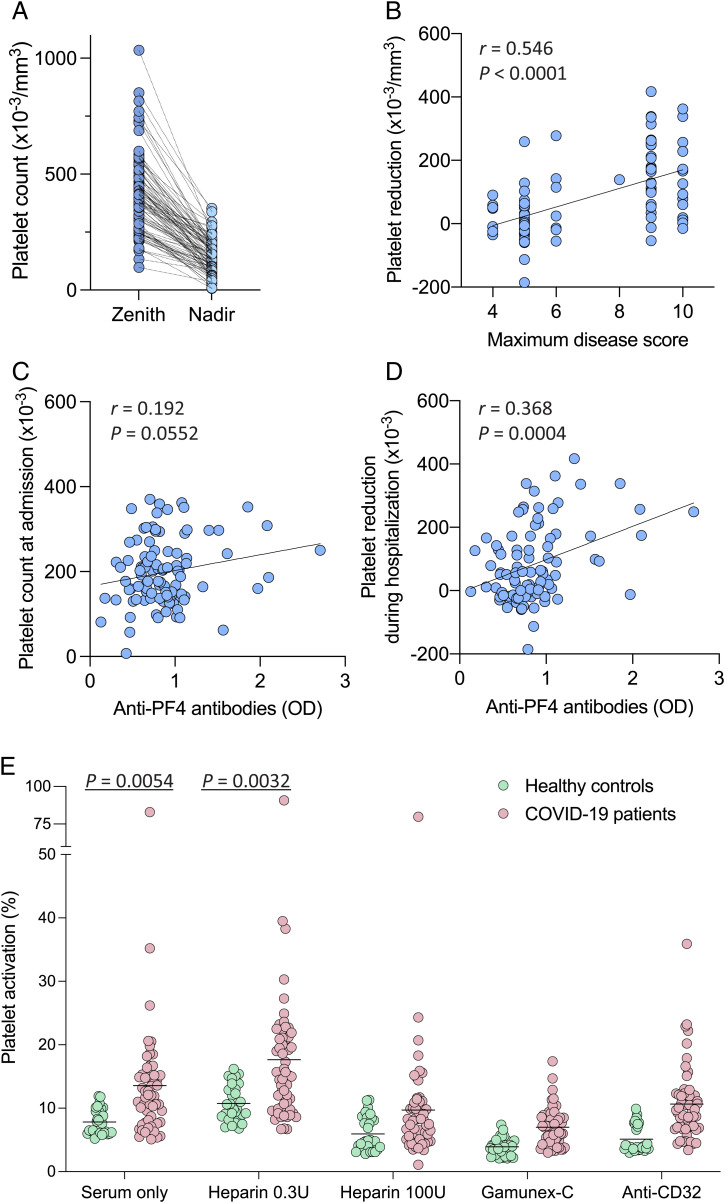
Fig. 4.
Anti-PF4 antibodies, circulating platelet counts, and ability to induce platelet activation in hospitalized patients with COVID-19 and controls. (A) Maximum (zenith) and minimum (nadir) circulating platelet counts in individual patients with COVID-19 during their hospitalization period (n = 100). The statistical difference was calculated by paired, two-tailed t test. (B) Linear correlation between platelet reductions and maximum disease severity score in patients with COVID-19 during hospitalization. (C) Linear correlation between anti–PF4 antibody levels and platelet counts in patients with COVID-19 at the time of hospital admission. (D) Linear correlation between anti–PF4 antibody levels in patients with COVID-19 and platelet reductions during hospitalization. Platelet reductions were calculated as the difference between the maximum platelet count recorded before the sampling date and the minimum value within a period up to 30 d after the sampling date. Statistical associations in B–D were evaluated using linear regression. Pearson’s correlations and P values are shown along with the fitted regression lines. (E) Platelet activation induced by sera from hospitalized patients with COVID-19 (n = 57) vs. healthy blood donors (n = 30). Platelet activation was measured by surface expression of P-selectin (CD62P). The tests were performed in the presence or absence of low-dose heparin (0.3 U/mL) as a stimulant, or high-dose heparin (100 U/mL), concentrated human Igs (Gamunex-C), or an anti-CD32 blocking antibody as inhibitors. Statistical differences for the indicated comparisons were calculated by unpaired, two-tailed t tests.