Figure 7.
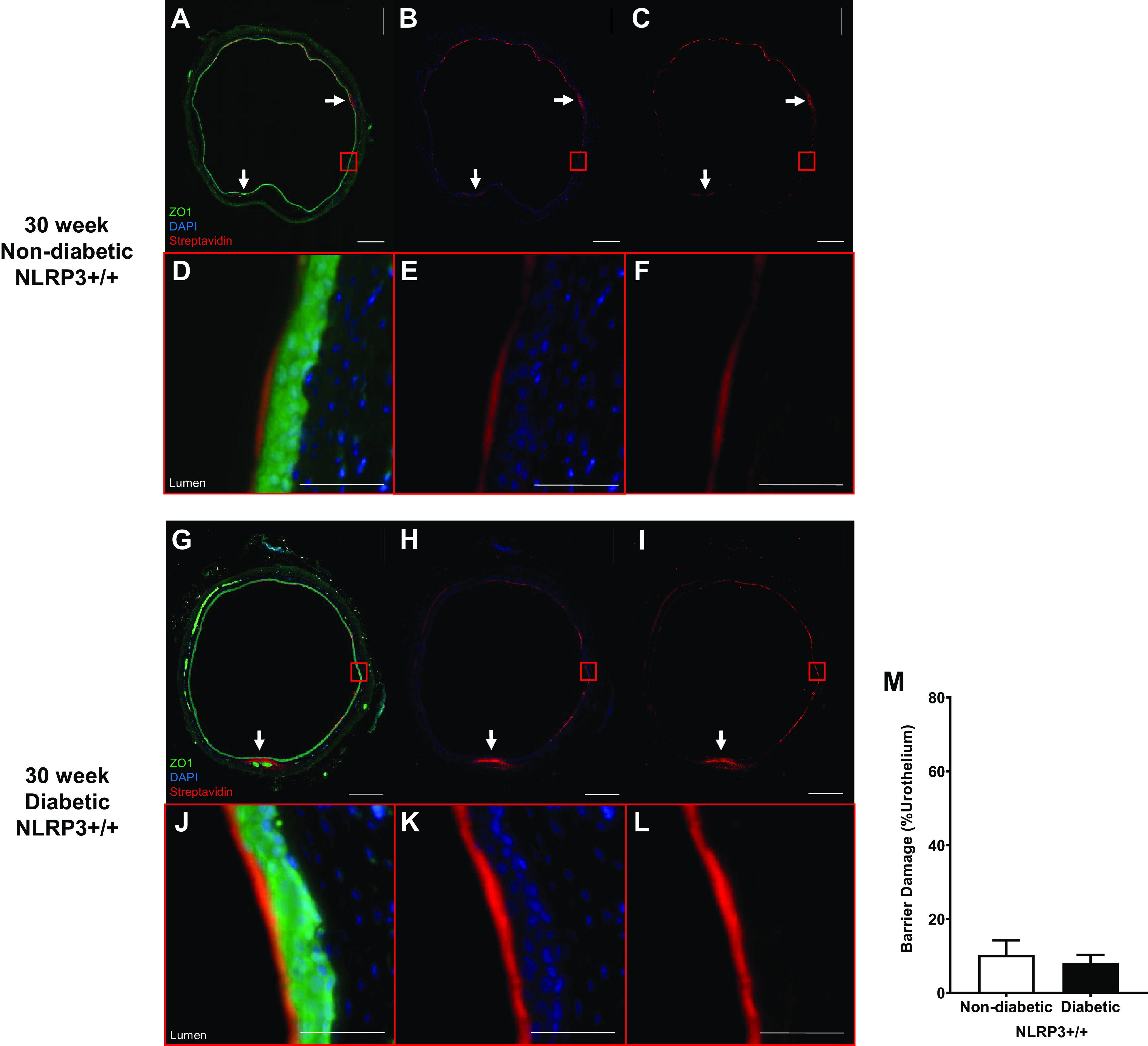
Diabetes does not impact urothelial permeability to sulfo-NHS-biotin at the 30-wk (underactive detrusor) time point. Sulfo-NHS-biotin was dissolved in PBS (1 mg/mL) and delivered intravesically (150 μL) through catheters placed in anesthetized mice. The sulfo-NHS-biotin solution was removed after 30 min and replaced with 150 μL of 4% paraformaldehyde. Bladders were tied proximal to ureters, excised, and paraformaldehyde fixed while inflated. Paraffin-embedded sections (5 µm) were then dehydrated and labeled with primary antibody to zona occludens (ZO1) to identify urothelial cells (shown as bright green), Texas red-conjugated streptavidin for sulfo-NHS-biotin detection (red), and DAPI for nuclei (blue). Regions of urothelia (ZO1, green) considered damaged contained sulfo-NHS-biotin (streptavidin, red) in the submucosa. Representative images of bladder sections are shown. White scale bars in A–C and G–I = 500 μm. In A–C and G–I, red boxes indicate select representative regions that are magnified in D–F and J–L, respectively. Scale bars in in D–F and J–L = 50 μm. A–F: 30-wk nondiabetic NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3)+/+ urothelial barriers were nearly fully intact with sulfo-NHS-biotin staining on the apical surface. Limited regions of damage in which sulfo-NHS-biotin has traversed the urothelia are indicated by white arrows. G–L: similarly, 30-wk diabetic NLRP3+/+ urothelial barriers were nearly fully intact with limited regions of damage in which sulfo-NHS-biotin has traversed the urothelia indicated by white arrows. M: no statistically significant changes in barrier permeability between diabetic and nondiabetic urothelia were evident at 30-wk time point. n = 3 and 4 animals, respectively. Statistical analysis was determined by a Student’s t test.
