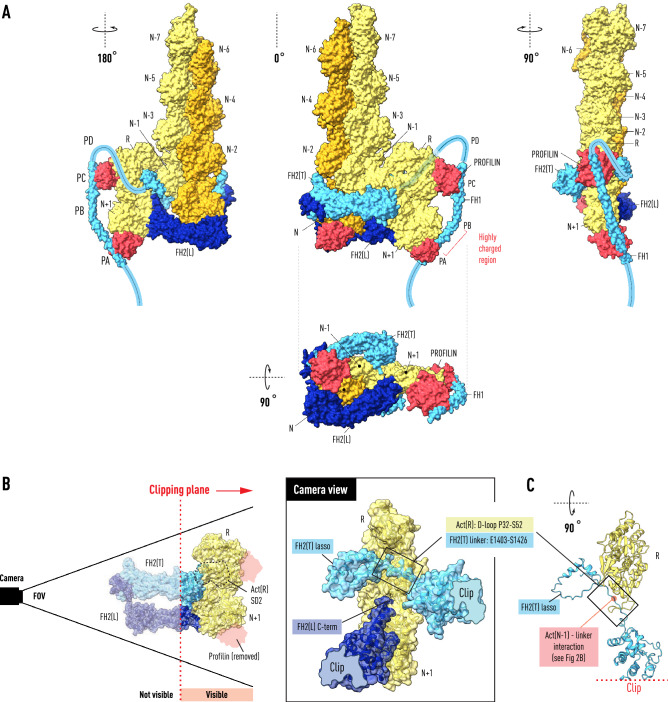
Figure 4.
Transition State for the Formin Elongation Reaction. The Bni1:actin FH2 heterodimer with bound F-actin (left in Fig. 1) is shown with a profilin–β-actin dimer derived from the crystal structure (space group 19; PDB: 2BTF) docked onto the penultimate actin(N−1) subunit. The center panel in (A) illustrates how the binding of the dimer positions actin(N+1) at the entrance to the polymerase at the open end with the axial spacing (5.5 nm) of the actin helix. The PA and PC poly-l-proline tracts of FH1 have been docked onto profilins bound to actin(N+1) and actin(R) respectively. The path (faint blue, middle panel) of the FH2(T) linker (1400–1420) in the open space below the actin(N−1)–actin(R) interface is dictated by its interaction with the actin(N−1) N-terminus, positioning the lasso on the opposite side of the dimer from its knob domain. Panel (A) show in three orientations a possible path for the FH1 polypeptide as it emerges (in the carboxy to amino direction) from the top of the FH2(T) lasso. Panel (B) shows the transition-state in the same orientation as the central panel of (A) but clipped by a plane the cuts through the center of the structure removing from view actin(N−1) and most of FH2(T) and FH2(L), except for parts of the knob and post domains respectively. The camera view (center), along a line perpendicular to the clipping plane, shows the space occupied by the FH2(T) linker and lasso. The negatively charged C-terminal α-helix of the post of FH2(L) is shown in the vicinity of the positively charged linker. Panel (C) shows selected residues of the clipped structure from the top to reveal the position (red dot) of a three-way interaction between the linker, the N-terminus of actin(N−1) and the D-loop of actin(R).