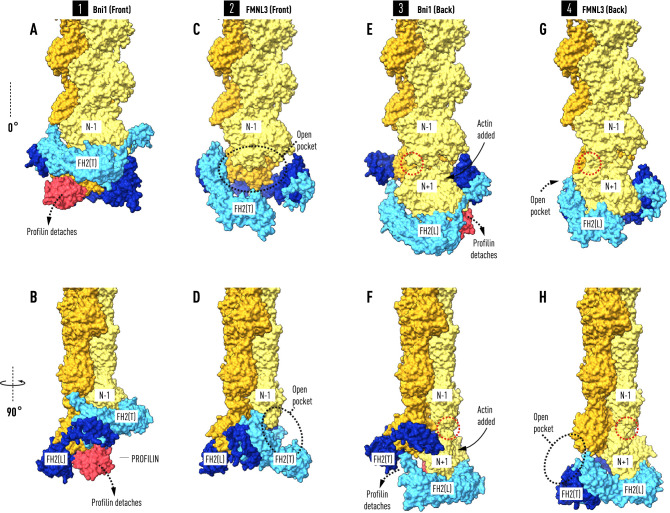
Figure 5.
Profilin controls the timing of the elongation reaction. The front and back views of the two co-crystal structures afford views of the interaction between FH2 domains and actin at four steps (A–B, C–D, E–F, G–H) during the elongation reaction. Panels (A) and (B) show two orientations of the Bni1 FH2-actin heterodimer (modeled as in Fig. 1) stabilized by the binding of profilin–actin(N) to FH2(L). Actin(N) and actin(N−2) are coplanar. In panels (C) and (D) profilin has been released from actin(N) during the migration of FH2(T) towards the barbed end creating a binding pocket for actin(N+1). Actin(N) rotates 13° and docks into the helical niche formed by actin(N−1) and actin(N−2). In panels (E) and (F) the insertion of profilin–actin(N+1) results in the coplanar alignment of actin(N+1) with actin(N−1). In panels (G) and (H), following the release of profilin, actin(N+1) rotates and docks onto the barbed end of the filament with the closing of a small gap (indicated by red dotted circle) between actin(N−1) and actin(N+1). Note that to model the four steps in the reaction sequence as viewed from the front, the colors of FH2(L) and FH2(T), as well as those of the filament strands, have been reversed in (E,F) and (G,H) from the front view structures on the left (A,B and C,D). To model the addition of the new subunit N+1, N (not labelled) in panels (A,B) and (C,D) is relabeled N+1 on panels (E,F) and (G,H).