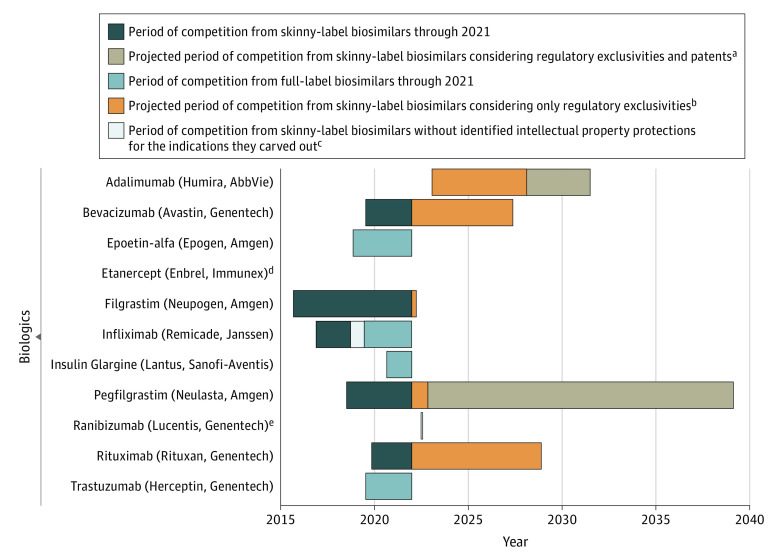
Figure 1. Competition From Skinny-Label and Full-Label Biosimilars for Biologics Approved by the US Food and Drug Administration (FDA) Through 2021.
aThis projected period of competition was based on the latest expiration date among patents and regulatory exclusivities protecting the indication(s) carved out of the biologic’s label.
bThis projected period of competition was based on the latest expiration date among regulatory exclusivities protecting the indication(s) carved-out of the biologic’s label.
cThis period of competition represents when only skinny-label biosimilars were commercially available but a full-label biosimilar could have been marketed based on the expiration dates of the identified patents and regulatory exclusivities protecting the carved-out indication(s).
dTwo full-label etanercept biosimilars were approved by the FDA but are not expected to be marketed until 2029.
eThis bar is shaded white.