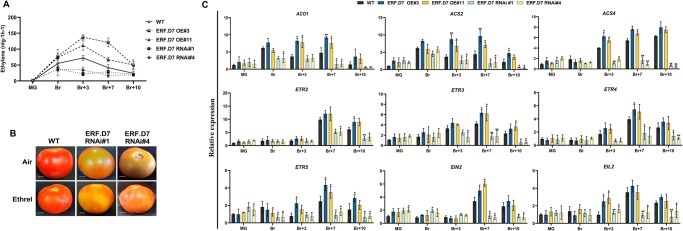
Figure 6.
Alterations in ethylene biosynthesis and perception in SlERF.D7 transgenic fruits. A, Ethylene production of WT and SlERF.D7 OE and RNAi fruits assessed at MG, Br, 3-day after Br (Br+3), 7-day after Br (Br+7), and 10-day after Br (Br+10) stages. Values represent the means of at least five individual fruits. The error bars represent ±sd of three biological replicates. B, Exogenous ethylene treatment on WT and SlERF.D7 RNAi fruit. MG fruits from WT and SlERF.D7 RNAi lines were injected with a buffer solution containing 10 mM MES, pH 5.6, sorbitol (3% w/v), and 100 µM ethrel (2-chloroethylphosphonic acid, 40% solution, SRL Diagnostics). After the treatment, fruits were incubated in a culture room at 26°C, under a 16-h light/8-h dark cycle with a light intensity of 100 μmol m−2 s−1 and photographed after 7 days. C, RT-qPCR analysis of ethylene biosynthesis and perception pathway genes at MG, Br, 3-day after Br (Br+3), 7-day after Br (Br+7), and 10-day after Br (Br+10) in SlERF.D7 OE, SlERF.D7 RNAi, and WT fruits with Actin gene as an internal control. Error bars represent ±sd of three biological replicates. Asterisks indicate the statistical significance using Student’s t test: *, 0.01 < P-value < 0.05; **, 0.001 < P-value < 0.01. ACO1, aminocyclopropane-1-carboxylic acid oxidase 1; ACS2 and ACS4, aminocyclopropane-1-carboxylic acid synthases; ETR2, ETR3, ETR4, and ETR5, ethylene receptors; EIN2, ethylene signaling protein; and EIL2, protein.