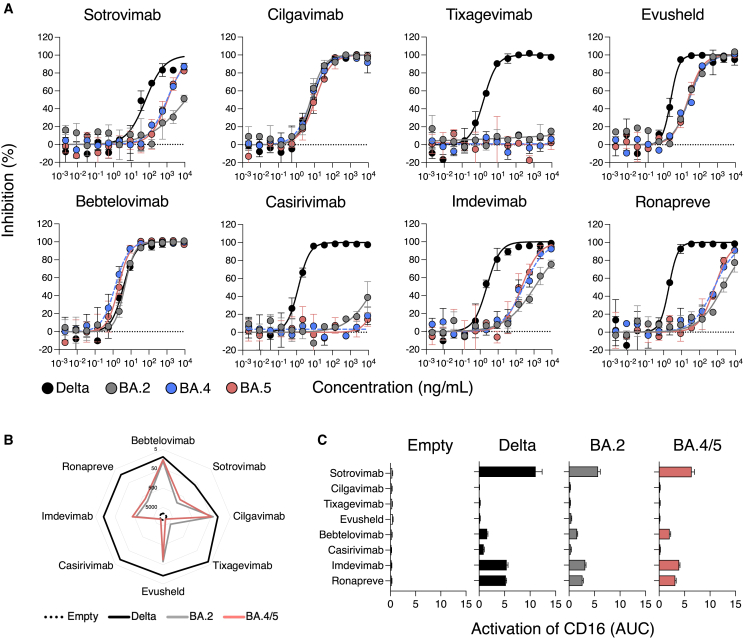
Figure 1.
Neutralization and antibody-dependent cellular cytotoxicity of Omicron BA.4 and BA.5 by therapeutic mAbs
(A) Neutralization curves of mAbs using the S-Fuse system. Dose-response analysis of the neutralization by the indicated antibodies and by Evusheld, a combination of cilgavimab and tixagevimab, and Ronapreve, a combination of casirivimab and imdevimab. Data are mean ± SD of 2 independent experiments. The IC50 values for each antibody are presented in Table 1. The dashed line indicates the limit of detection.
(B) mAbs binding at the surface of Raji cells stably expressing the indicated spikes. Raji cells transduced with a control empty vector not coding for any spike (Empty). Depicted are EC50, calculated with a curve fitting the percentage of mAb-positive cells measured by flow cytometry against antibody concentration in limiting dilutions. Data are mean of 2 independent experiments. The EC50 values for each antibody are also presented in Table 1.
(C) Activation of the CD16 pathway as a surrogate of the capacity of each mAb to elicit antibody-dependent cellular cytotoxicity (ADCC). The area under curve of a dose-response analysis of CD16 activation by each mAb against each SARS-CoV-2 variant is depicted. Data are mean ± SD of 2 independent experiments.