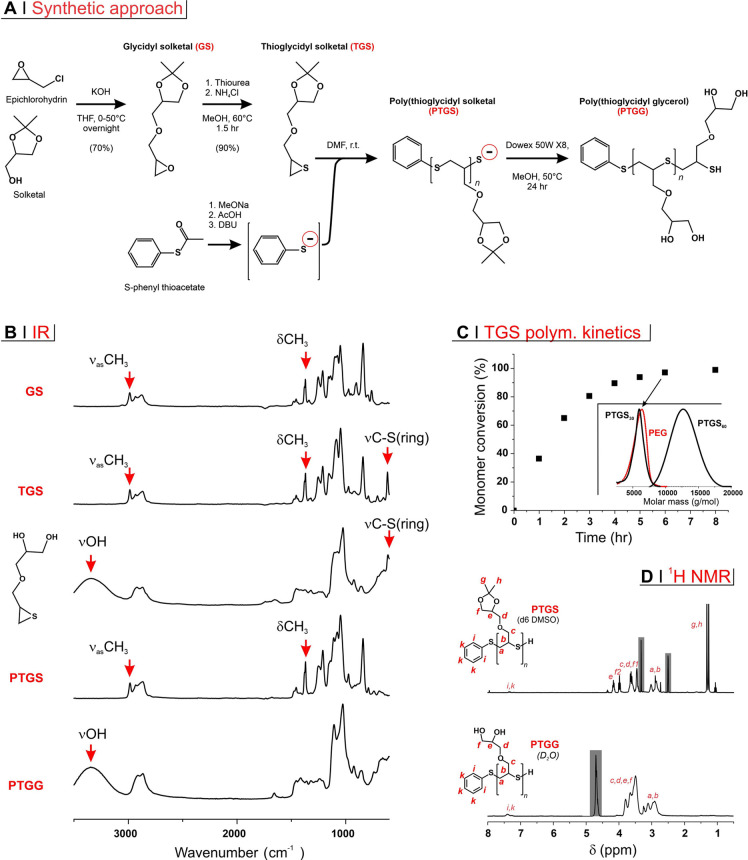
Figure 1.
(A) TGS was produced via a two-step reaction sequence and then used in the episulfide ROP initiated by an in situ formed phenyl thiolate; the resulting PTGS was treated with an acidic resin, to deprotect glycerol side chains and protonate its terminal thiol thereby producing the final PTGG. (B) IR spectra of compounds prepared during PTGG synthesis; episulfide rings can be monitored through the typical C–S stretching vibration of three-membered rings located at ∼600 cm–1. Glycol deprotection (both in the low molecular weight TGS and in the polymeric PTGS) corresponds to the loss of the methyl stretching and bending vibrations of the isopropylidene group and to the appearance of an OH stretching vibration. (C) TGS consumption kinetics as determined by 1H NMR in deuterated-DMF (reduction in intensity in the resonance at 2.90–2.92 ppm of episulfide ring protons [−CH2S−]CH−); theoretical DP = 30). In the inset, GPC traces (RI signal, triple detection in THF); the sample collected after 6 h of polymerization (PTGS30) is highlighted with an arrow; the other two refer to a 12 h of polymerization with a theoretical degree of polymerization = 60 (PTGS60) and to mPEG thioacetate; the latter and PTGS30 have very similar molecular weight distributions. (D) 1H NMR spectra of PTGS30 in deuterated DMSO and PTGG30 in D2O; letters correspond to the assignments on the chemical structures and shadowed areas to solvent peaks.