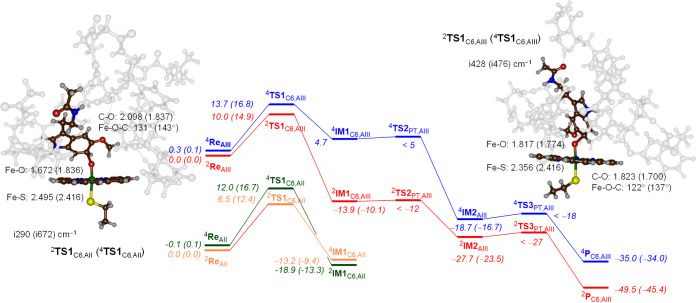
Figure 6.
Reaction energy profile for aromatic hydroxylation of melatonin using a CpdI model of CYP1A1 with the substrate in binding orientation II and III. Energies represent UB3LYP/BS3//UB3LYP/BS1+ZPE + Esolv data in kcal mol–1, while free energies are shown in parenthesis. Optimized geometries of the rate-determining step give bond lengths in angstroms, bond angles in degrees, and the imaginary frequency in cm–1.