Figure 3.
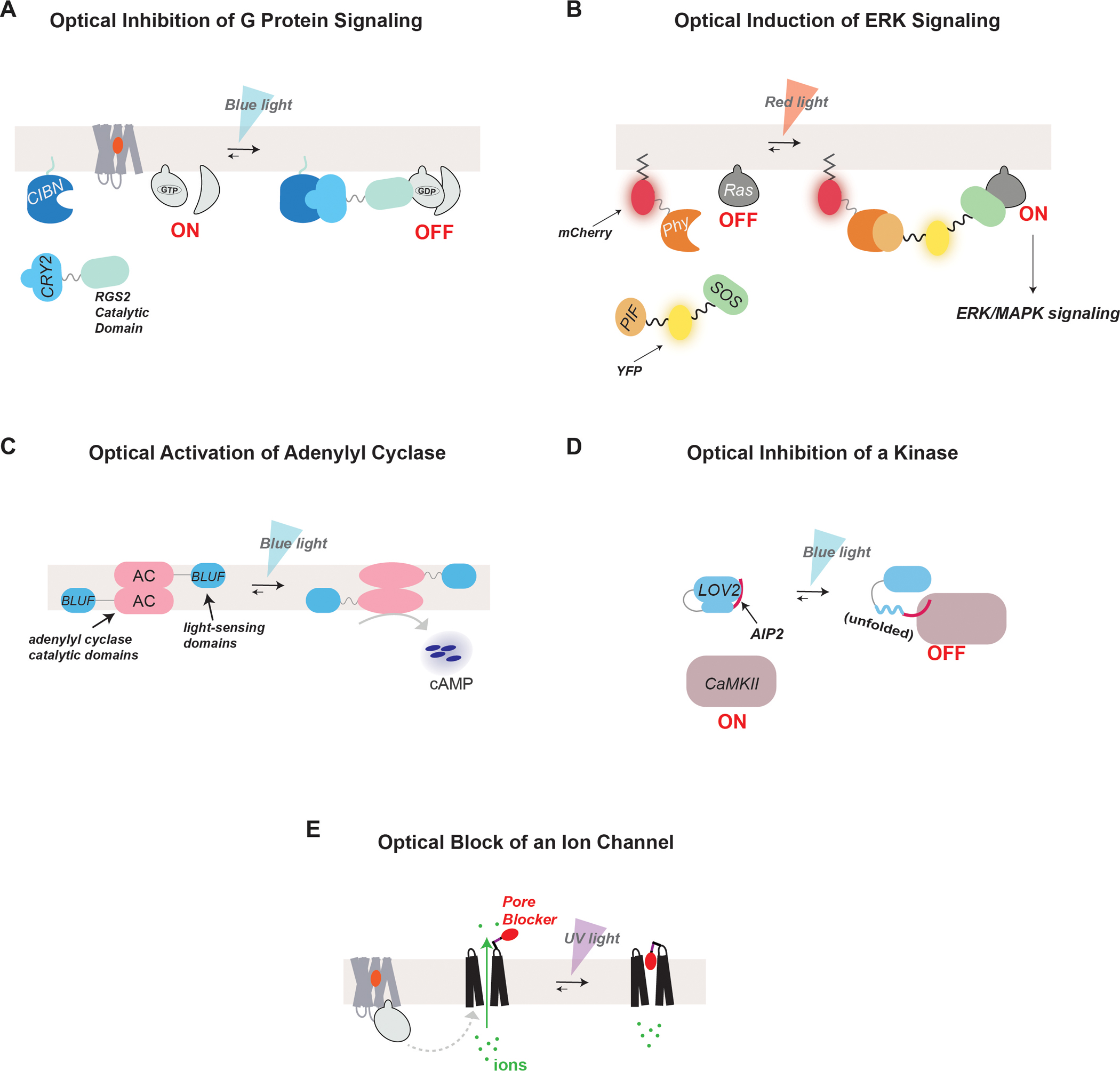
Optical methods for the control of GPCR effectors
(A) The CRY-CIB system can be used for blue light-induced association of two proteins. For example, optical inhibition of G protein-mediated signaling has been achieved by utilizing the CRY-CIB system to induce the translocation of RGS2 to the plasma membrane with blue light. This recruitment brings RGS2 in close proximity to membrane-bound Gα-GTP which allows it to effectively inhibit G protein signaling.
(B) The Phy-PIF system can be used for red light-induced association of two proteins. For example, photocontrol of ERK signaling can be achieved through the induction of the surface translocation of SOS using the Phy-PIF system. Phy was fused to a CAAX motif for membrane incorporation, while SOS was fused to PIF. Red light induces dimerization of Phy and PIF, translocating SOS to the membrane to promote interaction with and activation of Ras GTPase and initiate ERK signaling. Fluorescent proteins were incorporated into the constructs to observe their expression and dynamic localization.
(C) Optical control of cAMP production can be achieved by heterologously expressing bPAC, a photoactivatable adenylyl cyclase from Beggiatoa, which has increased catalytic activity in the presence of blue light.
(D) The LOV domain has been utilized to gain photocontrol of the activity of various signaling proteins. Following blue light illumination, the Jalpha helix of the LOV domain unfolds allowing it to release functional peptides or proteins for light-gated activity. In the illustrated case, LOV2 was fused to a CaMKII inhibitory peptide, AIP2, which becomes released in the presence of blue light.
(E) Photoswitchable tethered ligands can be employed for the optical control of ion channels. For example, a photoswitchable pore blocker may be attached to an ion channel using cysteine chemistry. The pore blocker is designed such that it fits into the opening of the channel only in the presence of light to effectively block ion conductance.
