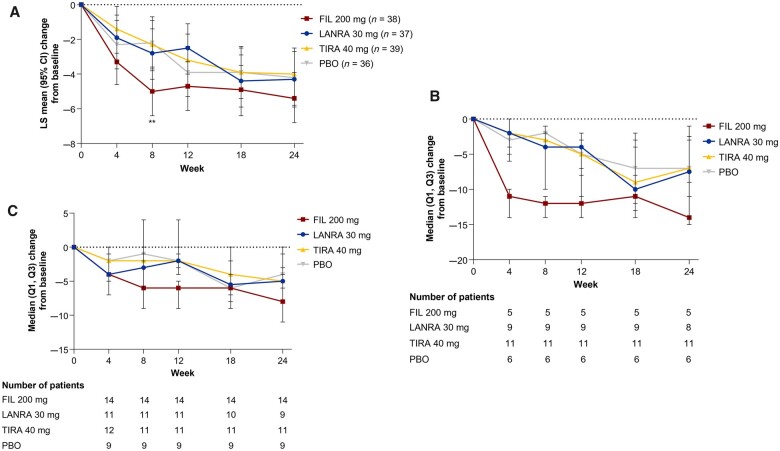
Fig. 3.
ESSDAI score–adjusted mean change from baseline
(A) Overall study population. Error bars show 95% CI of LS mean. LS means, 95% CIs and P values were obtained from a mixed-effects model for repeated measures with the terms for baseline value, treatment, stratification factors, visit, and treatment-by-visit interaction. **P < 0.01 vs placebo. The baseline value was the last available value collected on or prior to first dose of the study drug (day 1). (B) ESSDAI score–adjusted mean change from baseline in patients with baseline ESSDAI ≥14. Markers indicate median; error bars indicate interquartile range. Numbers below the graph show numbers of patients. The baseline value was the last available value collected on or prior to the first dose of the study drug (day 1). (C) The ESSDAI score–adjusted mean change from baseline in patients not taking DMARDs/CSs at baseline. Markers indicate median; error bars indicate interquartile range. Numbers below the graph show numbers of patients. The baseline value was the last available value collected on or prior to the first dose of the study drug (day 1). ESSDAI: EULAR SS disease activity index; FIL: filgotinib; LANRA: lanraplenib; LS: least square; PBO: placebo; Q1: first quartile; Q3: third quartile; TIRA: tirabrutinib.