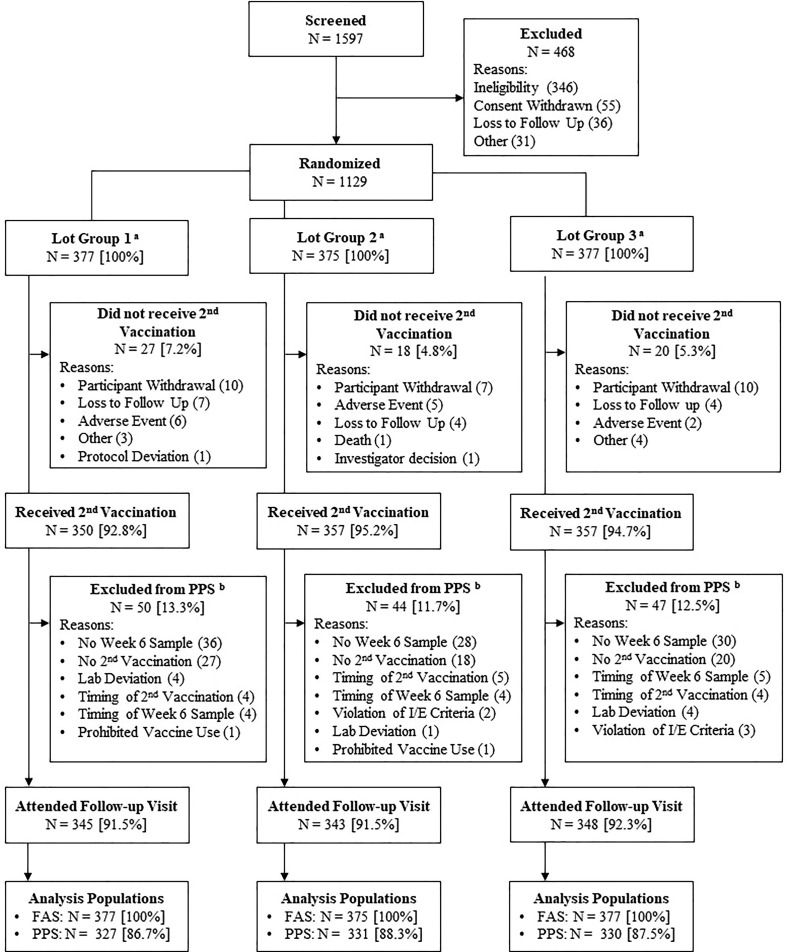
Fig. 1.
Subject Disposition (All Participants) Abbreviations: FAS = full analysis set; I/E = inclusion/exclusion criteria; PPS = per protocol set. Note: Participants excluded on account of timing were either vaccinated or had serum draws at timepoints substantially outside the timeframe specified in the protocol. a All randomized participants received the first dose of trial vaccine and were included in the FAS for the purpose of evaluating safety. b Some participants may have been excluded from the PPS for more than one reason; thus, the individual reason counts may add up to be more than the total number of participants excluded.