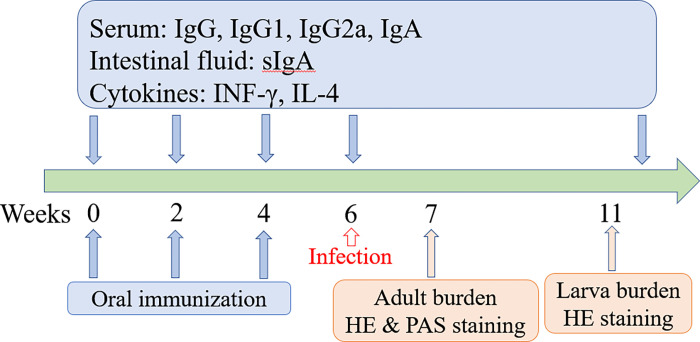
Fig 1. The vaccination scheme and detection protocol designed in this study.
Oral vaccination of mice with DNA vaccine was administered three times (weeks 0, 2 and 4). Five mice of each group were euthanized at week 0, 2, 4 and 6 after vaccination, levels of intestinal sIgA and cytokines (IFN-γ and IL-4) were determined by ELISA. The vaccinated mice were orally challenged with 300 T. spiralis ML two weeks following the final vaccination. At weeks 7 and 11 after vaccination (e.g., 7 and 35 days after challenge), ten mice of each group were sacrificed and intestinal adult worm, female fecundity and muscle larval burden (larvae per gram, LPG) were assessed to evaluate the protective efficacy induced by vaccination with DNA vaccine. Pathological changes of intestines and muscles from infected mice were examined under microscopy at 7 and 35 days after challenge.