Table 1.
Initial studies of carbene insertion into the N-H bond of N-Me aniline catalyzed by (Me)IrCYP119.a
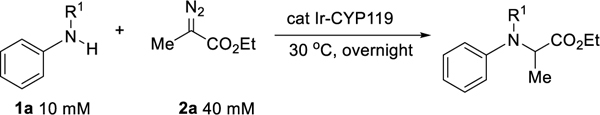
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | R1 | Catalyst | erb | Yieldb | TONc |
|
| |||||
| 1 | H | Purified ArM | 50:50 | 92% | 1840 |
| 2 | Me | Purified ArM | 40:60 | 56% | 1120 |
| 3 | Ac | Purified ArM | ND | <5% | -- |
| 4 | Ts | Purified ArM | ND | <5% | -- |
| 5 | Me | Cell lysis ArM | 50:50 | 54% | 1080 |
| 6d | Me | whole cell | 39:61 | 59% | 5867 |
| 7e | Me | whole cell | 45:55 | 23% | 2257 |
| 8f | Me | whole cell | ND | <5% | -- |
| 9g | Me | whole cell | ND | <5% | -- |
Reaction conditions: 2.5 μmol aniline derivatives, 10 μmol Me-EDA, 0.05 mol% Ir(Me)-MPIX with the CNH-0 mutant (155F, 213G, 254L, and 317G), 0.25 mL M9-N buffer, 30 °C, overnight.
The er and yield were determined by chiral HPLC with N,N-diethylaniline as the internal standard.
The TON was calculated by the number of substrate molecules converted to the product per iridium cofactor added to the cell culture.
The reactions were run in whole cells at an OD ~ 25 coexpressing the hug operon and CYP119 and 0.01 mol % Ir(Me)-MPIX.were
The reactions run in whole cells with only CYP119 expression.
The reactions run in whole cells with coexpressing the hug operon and a blank 2BT vector.
The reactions were run in whole cells coexpressing the hug operon and CYP119 but without the addition of Ir(Me)MPIX.
