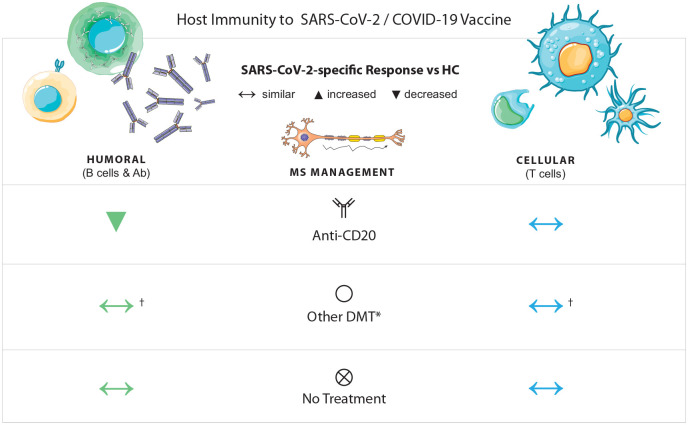
Figure 1.
SARS-CoV-2-specific humoral and T cell responses in patients with MS.
*Other DMT refers to GA, IFN-β, teriflunomide, fumarates, cladribine, natalizumab, alemtuzumab, and S1P modulators.
In patients with MS, humoral and cellular immune responses to SARS-CoV-2 infection and COVID-19 vaccination depend on the DMT. Patients with MS on RTX, OCR, OMB, or S1P receptor modulators exhibit decreased SARS-CoV-2-specific humoral responses compared with healthy controls. RTX-, OCR-, and OMB-treated patients still mount SARS-CoV-2-specific T cell responses, but S1P-treated patients may have reduced T cell responses. Patients with MS on other DMTs, including GA, IFN-β, teriflunomide, fumarates, cladribine, natalizumab, and alemtuzumab, also generate virus-specific T cell responses. Treatment with RTX, OCR, OMB, or non-S1P-modulating oral therapies was associated with enhanced T cell response after a two-dose vaccination with a mRNA COVID-19 vaccine.
Ab: antibody; COVID-19: coronavirus disease 2019; DMT: disease-modifying therapy; GA: glatiramer acetate; HC: healthy control; IFN: interferon; OCR: ocrelizumab; OMB: ofatumumab; RTX: rituximab; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; S1P: sphingosine 1-phosphate.