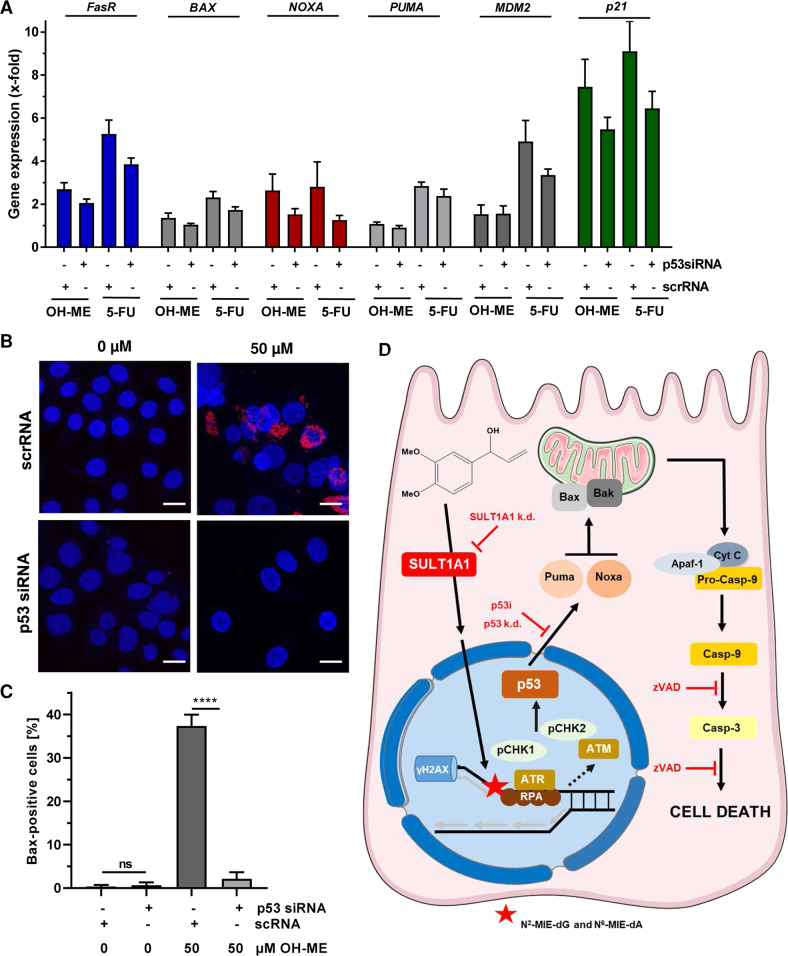
Fig. 6. Impact of p53 on pro-apoptotic gene expression and Bax activation upon ME-derived DNA damage.
A Expression of pro-apoptotic genes (NOXA, PUMA, BAX, FASR) and other p53-related genes (p21, MDM2) in HepG2 cells after p53 knockdown and treatment with 75 µM OH-ME for 24 h. Gene expression was assessed by qPCR (n = 4). Data are expressed as mean + SEM. B Assessment of Bax activation in HepG2 cells following p53 knockdown and treatment with 50 µM OH-ME for 48 h. Cells were fixed, processed, and immunostained for activated Bax (red), while nuclei were visualized by DAPI. Images were acquired by confocal microscopy. Scale bar: 20 µm. C Quantitative evaluation of Bax-positive cells (n = 3). Data are shown as mean + SEM. Ns: not significant, ****p > 0.0001. D Model of ME-triggered replication stress, DNA damage response, and cell death induction via p53 in liver cells. ME causes N2-MIE-dG and N6-MIE-dA adducts via its primary metabolite OH-ME, which is activated by sulfate conjugation catalyzed by SULT1A1. Genetic knockdown of SULT1A1 or low intrinsic SULT1A1 levels strongly attenuate DNA adduct formation, highlighting its critical role. The induced DNA adducts cause replication stress as evidenced by γH2AX formation and CHK1 phosphorylation. ATR-CHK1 activation together with delayed CHK2 activation, presumably catalyzed by ATM, lead to p53 stabilization. The tumor suppressor protein then triggers a cell death program via upregulation of the BH3-only factors PUMA and NOXA, which result in Bax activation and cytochrome c release from mitochondria. This causes apoptotic cell death via caspase-9 and caspase-3 cleavage, which is rescued by the pan-caspase inhibitor zVAD. Importantly, this cell death cascade is driven by p53 as demonstrated by pharmacological p53 inhibition or its genetic ablation. This figure was created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com.