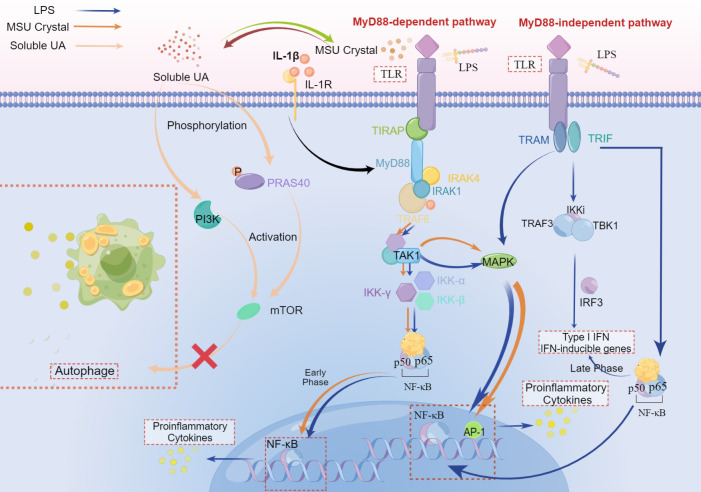
Figure 3.
The crosstalk of LPS, MSU crystal, and soluble UA in immune-inflammatory signal pathway. Both MSU crystal and LPS could activate TLRs to recruit TIRAP and MyD88. The stimulation of MyD88 molecule by ligands primes the interaction of MyD88 and IRAK-4, which allows IRAK-1 and TRAF-6 to associate with MyD88 and subsequently recruits TRAF6, leading to the activation of TAK1 to stimulate MAPK that upregulates the inflammation responses via AP-1. Followed by activation of TAK1, the IKK complex (inhibitor of nuclear factor-κB (IκB)-kinase complex), which consists of IKK-α, IKK-β, and IKK-γ, translocates NF-κB to the nucleus, increasing the pro-inflammatory cytokines in the early phase, pro-resolution cytokinesis in the late phase. This is a MyD88-dependent pathway. In the MyD88-independent pathway, TLR is activated by LPS and then recruits TRAM and TRIF which induces the combination of TRAF3, TBK1, and IKKi to activate IRF-3, which plays a critical role during the late phase of NF-κB activation (30), aiming at Type I IFN and IFN inducible genes. In this pathway, the translocation of NF-κB can both elevate levels of pro-inflammatory cytokines and IFN. In addition, MAPK can also be activated after the recruitment of TRIF and other adaptors. IL-1β binding to IL-1R plays an accelerating role in the MyD88-dependent pathway. Soluble UA phosphorylates PRAS40, leading to the activation of mTOR, which can also be realized via PI3K. While both PRAS40 and mTOR inhibit autophagy, suppressing the inactivation of inflammatory cytokines.