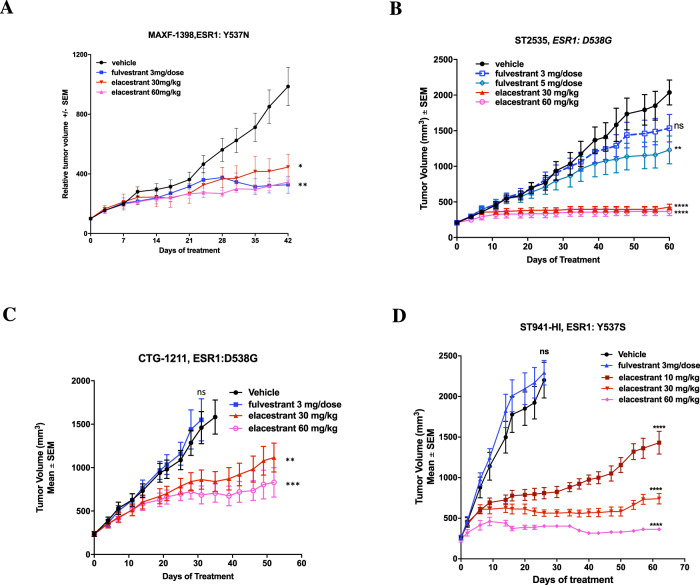
Fig. 5. Effect of elacestrant versus fulvestrant on tumour progression in endocrine-resistant PDX models.
A Long-term study assessing changes in tumour volume over 42 days of treatment in MAXF-1398, an ER+ model harbouring an ESR1Y537N mutation and previously exposed to radiation and tamoxifen therapy but sensitive to fulvestrant (n = 5–6 animals/arm). B, C Long-term study assessing changes in tumour volume of treatment in B ST2535-HI (n = 7–10 animals/arm) and C CTG-1211-HI (n = 9 animals/arm) PDX models that are ER+ harbouring ESR1D538G mutations and previously exposed to tamoxifen, AI and fulvestrant therapy. D Long-term study assessing changes in tumour volume over 64 days of treatment in ST941-HI, an ER+ model harbouring an ESR1Y537S mutation and previously exposed to fulvestrant (n = 7–10 animals/arm). Mice were treated with vehicle control, fulvestrant (3 mg/dose) or elacestrant (10 mg/kg, 30 mg/kg or 60 mg/kg), as indicated. Data represent mean relative tumour volume (mm3) ± SEM. ANOVA with a Dunnett’s post-test was carried out for group comparisons. p values *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.