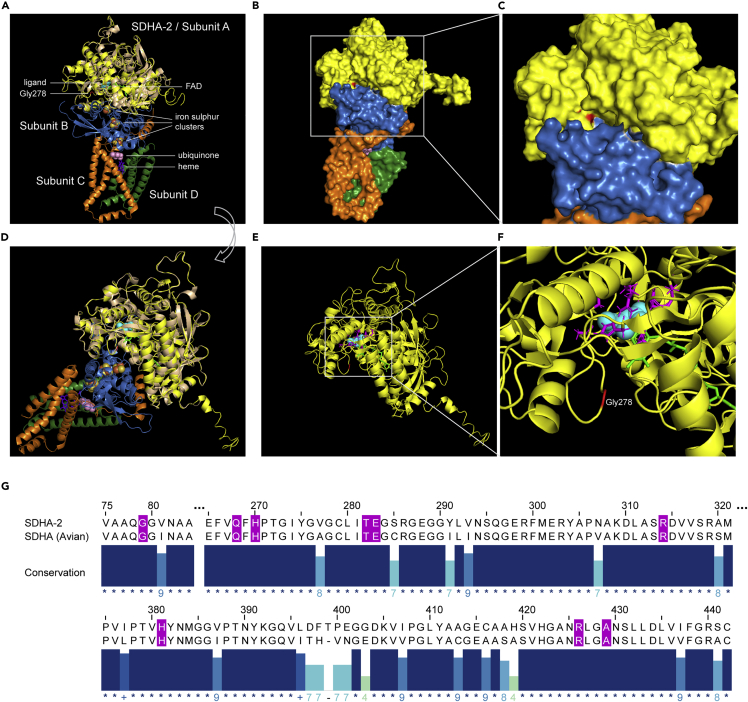
Figure 2.
SDHA-2 protein model
(A–F) I-TASSER-generated model of SDHA-2. (A–C) show the complex from a perspective highlighting the 4 subunits; (D–F) show the protein/complex from a different perspective highlighting the ligand binding site. The SDHA-2 model is colored yellow and SDHA-2 Gly278 is colored red. (A and D) Two perspectives of the SDHA-2 model aligned with PDB structure PDB: 1YQ3, Avian respiratory complex II with oxaloacetate and ubiquinone (subunits A-D) (Ref.22) 1YQ3 Subunit A is beige, B is blue, C is orange, and D is green. Subunit A binds a malate-like ligand (cyan balls) and FAD cofactor (green sticks). Subunit B is an iron-sulphur protein binding three different iron-sulphur clusters (yellow and orange balls). Subunits C and D bind a heme molecule (purple sticks). Ubiquinone is represented by pink balls. (B and C) Surface model of SDHA-2 and 1YQ3. SDHA-2 Gly278 (red) is near the predicted interface with subunit B (blue). (E and F) The SDHA-2 model alone. Side chains of the residues comprising the predicted malate-like ligand binding site (from homology with Avian respiratory complex II subunit A (Ref.22) are colored magenta.
(G) Amino acid sequence alignment of regions of SDHA-2 to Avian SDHA using Clustal Omega. Numbering refers to SDHA-2 residues. Residues comprising the predicted malate-like ligand binding site are colored magenta (Ref.22)